|
Deutsches Rheuma Forschungszentrum Berlin, Robert
Koch Institute, Haus 11, Nordufer 20, D-l000 Berlin 65 This paper tries to put recent work from my laboratory into the wider context of the use of peptides in T -cell biology. The names of my old colleagues in London can be found in the references (Villarreal et al ]990, Robertson et a] 1992), as also can those of my new ones in Berlin (Robertson et a11992, Brunner et a] 1992), who did the work. In truth our contribution to this burgeoning field has been slight, although we have enormously enjoyed playing a small role in a great enterprise. It is one which is still gathering speed. Perhaps this account will give some idea of activity which is moving from laboratory studies into diagnostics, and on from there on into therapy. The trial of orally administered type II collagen in rheumatoid arthritis which my colleague Dr. J.S. Sieper and I have been planning has been a great stimulus to thought. The title here is misleading in so far as advances in understanding the trimolecular interaction between MHC molecule, peptide, and T -cell receptor are not covered. They make up another whole branch of the subject, well reviewed by Rothbard and Gefter ( 1991 ).
An epitope, by definition, is that part of an antigen which is recognized by a lymphocyte. Luckily this discussion is confined to T -cells, where the question of what is recognized can be answered very simply, as has been discovered only quite recently (reviewed in Rothbard and Gefter 1991). In brief, major histocompatibility molecules (MHC) of both class I and II type have a groove that accommodates peptide nonamers (i.e. sequences of nine amino acids), and it is these nonamers (plus the surrounding MHC structure) that are recognised by the antigen-specific receptors (TcR's) of T -cells. A nonamer constitutes the "epitope" or determinant part of an antigen which is recognised by T -cells. In principle it is possible simply to scan epitopes in an entire protein by means a set of "nested" (overlapping) nonamers, but for most proteins this would be prohibitively expensive. Rules for predicting epitopes have been formulated, but these cannot yet be relied on. An interesting question is why a length of exactly nine amino acids has been chosen. The hard nosed biochemist might argue that this size is dictated by the geometry of the MHC molecule. These molecules evolved from a single domain ancestor of immunoglobulin type, and no doubt there are limits to what members even of that super-family can accomplish. I doubt however whether those limits are tight enough to specify the length of the groove so precisely, and prefer a more evolutionary line of thought. It seems likely that the length reflects a balance of selective pressure between two opposing needs: on the one hand to conserve as much of the T -cell repertoire as possible, and on the other to prevent pathogens from building proteins invisible to the T -cell system. One can imagine primitive vertebrates trying out various lengths so as to find out which one suit them best, with the outcome shown in figure I. Perhaps the balance illustrated there could be tested against the nonamer outcome by calculation, provided that the number of amino-acid permutation allowed is known; pockets in the MHC structure specify restrictions, and so on. That expert modeler of the immune system Alan Perelson is tackling the problem, which is good news.
Hunting for epitopes is easy enough in principle. All that is needed is (1) a screening agent, normally a population of activated T -cells, (2) a screening procedure, such as a proliferation assay, and (3) a set of pep tides derived from the protein under study. As regards the screening agent, unselected populations of activated T -cells can of course be obtained from human disease patients or from immunized animals. Usually these are not suited to the task because of their low level of reactivity, although in our experience they give workable signals with exceptionally powerful microbial antigens (VillarrealRamos et a] 1991). In the mouse this problem can best be solved by making T-hybridomas, as will be mentioned in connection with our F liver protein study. Unfortunately this technology has not yet been developed for human T cells, which leaves a conspicuous gap in human immunology. Perhaps someone with experience of growing human T -leukaemia cells may read this paper, and come to the rescue. So instead, one can try to make use of antigen-specific human T -cell clones. That is a troublesome business, and introduces an element of selection while the clones are being picked. Perhaps the best thing is to compromise, and flit to and fro between mouse and man trying to spot epitopes which work in both species. This in fact was what was done in the lead study on HIV epitopes that is discussed below. As regards screening procedures, proliferation assays no doubt set the standard. Reactions of T -hybridomas have to be assayed by IL-2 release, and even with unselected T cells the trend is towards measuring cytokine release rather than proliferation. IFNy is sometimes preferred to IL-2 for this purpose, as the assay has less problems with cytokine-consumption by receptors on activated T -cells. In no human autoimmune disease have the culprit T -cells been definitively identified. Accordingly methods are being developed for screening which do not depend on having specific T -cells. Thus my colleague Angelika Daser is scanning the 57 kD heat-shock protein of Chlamydia trachomatis for peptides able to bind to HLA B27, by means of a mutant B cell line provided by Dr Donald Pious (Seattle) that lacks the so-called "transporter" genes (alias "pump", "TAP", "ring", or " ABC" A TP-binding casette genes). These cells express low levels of MHC class I proteins because peptides are not transported across their endoplasmic reticulum and so cannot stabilise the nascent proteins. By providing extrinsic peptides the proteins can be stabilised and will then accumulate in increased quantity. Because HLA-B27 has been found guilty by association in several rheumatological diseases, and because Chlamydia induce B27-associated reactive arthritis, we plan to develop this screening procedure further. An important step will be to cross-test the peptides for activity in an in vitro competition assay with B-27-restricted T-cells specific for a third-party epitope (Guillet et aI1987). As regards making sets of peptides, mention has been made of the prohibitive expense of synthesising a complete set of nonamers in order to scan a protein. It seems better to start either with cleaved fragments of the protein (e.g. cyanogen bromide cleavage), or to take advantage of advances in biotechnology to make recombinant peptides. This has the advantage that one can start with large peptides, and then move on immediately to their smaller fragments. New England Biolabs sell an expression system using the E. coli maltose binding protein which is giving us excellent results. Peptides of 50-100 amino acids are well within its capability, although uniformly high yeilds are not expected. For experiments with definitive nonamers, chemical synthesis is faster, easier and cheaper than recombinant DNA technology. For epitope screening of larger, overlapping protein fragments however the maltose binding protein system seems to be extremely efficient. Starting from cDNA, in our case from that of type II collagen kindly given by Professor Vuorio (Turku), Roland Lauster, Eva Rajnavölgyi and Sinasi Gayrak have amplified those parts which encode the desired stretches of amino acids, using the polymerase chain reaction (PCR). The synthesis of specific 3' and 5' primers allows cloning of the amplified fragments into an E.coli plasmid vector, so that the collagen peptide is expressed as a fusion protein C-terminal to the maltose binding protein. In our first set of experiments the fusion proteins represent 20-40 % of the total E.coli proteins after chemical induction of gene expression. Further purification is achieved by the maltose binding capacity of the fusion product on an affinity column. The two fusion partners of the protein can subsequently be separated by the use of factor Xa proteinase. The corresponding recognition sequence has been constructed as a joining element by the manufacturers of the plasmid.
Most of the T -cell response normally focuses on a small number of epitopes on anyone protein; the control experiment with lysozyme in Lehmann et al (1992) provides a good example. Selection operates at various levels: during proteolytic cleavage within antigenpresenting cells, by MHC molecules, and within the T -cell repertoire as a result of negative selection. Perhaps competition between peptides for binding to MHC molecules is the point at which selection operates most stringently: "epitope dominance" is a term applied to the outcome of this competition. As is further discussed below, the dominant epitope does not entirely monopolise the response. There is scope for other epitopes to join in, and these additional epitopes can be exploited for diagnostic purposes as described below.
Single, intact proteins are widely used for exploring the T cell repertoire. Indeed exploring reactivity to such selfproteins as myelin basic protein, acetyl choline receptor, microsomal thyroid peroxidase and type II collagen has become something of a world-wide industry, as all of them are implicated one way or another in autoimmune disease. The problem is that although reactivity towards these proteins may rise in the course of disease, T -cells from normal individuals can also react, although usually to a lesser extent. Furthermore not all patients show heightened reactivity. One can argue to and fro. For instance, the level of reactivity found in healthy individuals can be dismissed as ineffective, and ascribed to activation by cross-reactive foreign antigens. In trying to sort matters out one runs into a technical difficulty that is inherent in any proliferation assay conducted with a single-protein antigen: there is a range of normal variation, which means that a cut-off has to be set at a moderately high level, so that weak positive responses tend to score as negative The problem of the single-protein assay has been attacked in a promising way in the field of Human Immunodeficiency Virus research. An important question is whether individuals are ever able to resist HIV infection. Accordingly, Berzowsky, Shearer and their colleagues constructed a set of six peptides from the viral envelope that could be recognized by T -cells from immunized mice and from HIV-infected human, of diverse MHC type (Berzowsky et al 1991). Reactivity towards these peptides was then examined in seronegative but high risk groups, such as homosexual men with recent exposure to HIV from their partners (Clerici et al 1992). This enabled a small but highly significant sub-group to be identified, who did react to the peptides but remained seronegative, suggesting that transient infection had indeed been resisted successfully. The reaction to the peptides was convincing precisely because individuals who reacted at all did so to several of the peptides. It is unlikely that such a firm conclusion could ever have been reached with a single-protein assay. This study also disposes of the reservation concerning peptide stimulation that it might not be possible to find peptides able to bind to the range of HLA molecules that occur in the human population. It is true that this study was carried out with peptides derived from a viral rather than an autoantigen. This in fact strengthens the argument, as viral peptides unlike autoantigens have no doubt been selected in evolution to avoid binding to HLA molecules. A third significant point that emerges from this study is that an excellent way of assessing the reactivity of T -cells from peripheral blood is by IL-2 release. This assay was tested extensively in parallel with a conventional proliferation (tritiated thymidine incorporation) assay (Clerici et al 199l), and has now become standard practice in that group.
When self-proteins are used to explore the T cell repertoire, as mentioned above, just what is being explored? Is only the repertoire of activated T -cells detected, or is it the entire repertoire? This important question has been largely answered by recent analysis of the T -cell subsets marked by different CD45R isoforms (reviewed in Brunner et al1992). It seems that expression of CD45RO marks an activated T -cell population, which gradually reverts to CD45RA expression and quiescence (this view is not universally accepted; Brunner et al draw attention to one crucial gap in the available information). Only .CD45RO T -cells can respond in a proliferation assay as It IS normally carried out, i.e. without costimulation. With costimulation, e.g. from a lectin, quiescent, CD45RA cells can also participate (Sanders et al 1989). According to this view there are two repertoires, one of which has almost entirely escaped attention: by Introducing costimulation the world-wide industry could double its output, and would then have more to tell us about the underlying nature of autoimmunity.
My colleague Susanne Schneider has discovered that immunization of mice with allo-F-liver-protein (immunogenic in vivo) raises a population of CD4 -cells identified via T -hybridomas as able to respond in vitro to self-F-liver-protein (although this protein appears to be completely non-.immunogenic in vivo). These may simply be THl cells, in contrast to TH2 cells which appear inactive in vivo,.a possibility that she is trying to test by manIpulatIons wIth IL-4. A more interesting possibility is that she may have come across an example of positive selection of the T -cell repertoire, of the type proposed by Cohen and Young (Cohen and Young 1991). Their immunological "homunculus" is a repertoire that is greatly expanded in certain areas, particularly by the potential autolmmunIty-inducers such as myelin basic protein and the others mentioned above. But they may not be the only inducers of positive selection, and in F liver protein we may have a different kind of positive selector. This possibility is supported by the old finding that this protein IS exceptionally Immunogenic. When mice are immunised with homogenates of one another's liver in Freund's adjuvant, F liver protein is the only allotype which generates precipitating levels of antibody. Generalising, the T -cell repertoire can be considered as having. a complex structure, with many centres of positive selection (contained, no doubt, by suppressive mechanisms) and also many holes created by negative selection. If one were to make a voyage of exploration starting from an epitope related to self, one would not know what to expect. The repertoire might be "trimmed" by self, to use Janet Maryanski's phrase, but equally it might be much expanded.
Thus far this discussion has concentrated on crosssectional analysis of the T -cell repertoire, i.e. what can be found if individuals are compared at anyone time, as in a case/control study. This sort of information can be enriched by horizontal analysis, in which changes in the repertoire of activated T -cells are studied over a period of time. As mentioned above, the T -cell response is highly restricted, so that to begin with only a few dominant epitopes are likely to be recognised. Powerful mechanisms then come into operation for "spreading" the response from one epitope to another through cell-cell interactions, both T -Band T -T, and both to and from CD4-cells (Mitchison 1990b). Lehmann et al (1992) propos.e that autoantigens may be particularly adept at spreading the response to "cryptic" epitopes, and that this may. constitute a key mechanism in generating autoimmune disease. This is a stimulating idea, although the evidence derived from their single EAE experiment in the mouse is a little thin. Once again Human Immunodeficiency Virus research points the way. Two groups have used peptide analysis to identify epitope dominance in the human cytotoxic T -cell response to the virus, and both have observed that the dominant epitope change over a period of months (Nixon et al 1.99 l ' Johnson et al 1992, plus personal Communications from both senior authors). There is some ambiguity in these data because of virus selection and the possibility of reinfection, but on the whole they suggest that epitope shifts occur fairly commonly during chronic Immunization.
As well as answering repertoire questions, analysis of the T -cell response can proceed to functional studies on what these cells do after activation. In man the possibilities are limited, and attention has focussed on the pattern of cytokine secretion. Hitherto the main subdivision has been into THO, THl and TH2 cells, in man mainly identified by secretion of IFNy versus IL-4. This classification, introduced originally in the mouse, applies on the whole well in man (Brunner et al 1992). However a fourth important category is now becoming evident, namely TGFß-secreting (transforming growth factor ß) T -cells originating in the mucosal tissues (Miller et al 1992) which is further discussed below. , Cytokine production patterns can of course be determined in the supernatants of stimulated T -cells, but a procedure applicable to single cells might be preferred, and would certainly provide additional information. An attractive technique involves paraformaldehyde-saponin fixation and cell-opening, followed by immunofluorescence analysis (Hallden et al 1989, Sander et al 1991). When used in conjunction with FACS analysis of surface activation markers, this technique offers the possibility of enumerating activated T -cells making particular cytokines. This technology has not yet entered general use, and still needs to be explored carefully. Many other functional assays are possible in animal experiments. These include ( 1) IL-2 secretion by T hybridomas, (2) helper activity for B-cells (Lightstone et aI1992), (3) desequestrating activity (Dietrich et aI1992), and (4) co-induction of autoimmune disease such as collagen-induced arthritis (T -cells used in conjunction with B-cells or anti-collagen antibodies). None of these procedures have progressed to the point where they need discussion here.
Single-epitope vaccines are attracting enormous interest because of their apparent simplicity and ease of manufacture by biotechnology methods. For example, the Walter Reed Army Institute for Medical Research still hopes to implement a malarial sporozoite vaccine, in an improved form in which a T-epitope would be combined with a B-epitope which on its own has given disappointing results. Vaccines of this sort are open to an objection which Barrie Bloom points out: they invite the parasite to escape by varying its target epitope. The Human Reproduction Programme of the World Health Organisation has long supported development of a single B-epitope vaccine for use in the only context where this problem does not apply, namely anti-selfvaccination. The epitope is located at the C-terminus of chorionic gonadotrophin, a hormone responsible for sustaining pregnancy, where it is hoped that antibodies could have a contraceptive effect. But even under these favorable circumstances it is by no means clear that the singleepitope vaccine will work as well as one based on the intact protein (Mitchison 1990a). Negative regulation of the immune response, for the purpose of intervention in autoimmunity and allergy, offers a greater hope of success. Peptides have repeatedly been shown to down-regulate the response in a highly specific manner, but there is much less agreement on just how they do so. The simplest possibility is that the peptide pre-empts the MHC molecule which would otherwise drive the response. This would be beneficial in autoimmune disease, if the blocked molecule would otherwise present a disease-inducing peptide to T -cells. Competitive blocking of this sort has been demonstrated in vitra (Guillet et al 1987), and systematic efforts have been initiated to implement this possibility in viva (Adorini et al 1988, Mc Devitt et al 1989). The great attractions of this approach are that most autoimmune diseases are driven only by a very limited number of MHC molecules (e.g. rheumatoid arthritis in northern Europe mainly by HLA DR4), so that the target for blocking is quite narrow; furthermore the target is a well defined molecule, whereas the target of approaches directed at T -cells themselves is undefined, at least in the human autoimmune diseases. The problem inherent in this approach is that blocking would be hard to maintain, as both the pep tides and their MHC targets have short half-lives. Accordingly interest is shifting towards longer-term effects, where Gefter's laboratory has been remarkably successful in obtaining long-term suppression of the immune response by subcutaneous injection of small quantities of T -epitope pep tides (Scherer et al 1989 and pers. comm.). A related study has been carried out on the prevention of collagen induced arthritis by peptide treatment; after much work this has culminated in the identification of a single active peptide (Myers et al 1992) .The question now is how these long-term effects work. A single petide that can suppress the presumably muti-epitope response to foreign collagen smells of bystander suppression, a mechanism discussed below. Gefter himself assigns peptide-mediated unresponsiveness to the category of "anergy", without worrying too much about the details of mechanism. Reading the papers of Gefter, or other recent publications on anergy (Ramsdell and Fowlkes 1992, Kang et al 1992, Mamalaki et al 1992) makes it all sound so simple. Negative selection (clonal elimination by apoptosis of reactive T cells) is what goes on in the thymus; unresponsiveness (other than blocking) induced in the periphery is mediated by anergy, a well-defined molecular lesion which is induced whenever the TcR is engaged in the absence of an appropriate costimulus. The lesion in the T -cells heals over a period of three weeks, unless renewed by further costimulus-free induction with the peptide or antigen. It is doubtful whether anergy is quite that ubiquitous. Indeed that understates the present position; I know of no evidence that normal (as distinct from super- ) antigens can induce anergy in viva under anything approaching physiological conditions. In our experience tolerance of soluble proteins has much the same properties, whether induced in the thymus or in the periphery (Robertson et al 1992), and there is little evidence that the affected T -cells can ever recover. On the contrary, this form of tolerance behaves as though mediated by clonal elimination. These doubts were confirmed by the account given by Philippa Marrack at the 1992 International Congress of Immunology, of unequivocal clonal elimination brought about by intravenous injection of superantigens; furthermore the doses required in her experience seemed very similar to those required to obtain tolerance of soluble proteins, whether in the thymus or in the periphery. So where does that leave the Gefter phenomenon? Only time and further experimentation can tell, but in the meanwhile one needs to keep an open mind. The issue is not trivial, because although induction of anergy and induction of peripheral clonal deletion may share features in common, notably the need to avoid costimulation, they would presumably have quite different stabilities. In passing, that fascinating T -cell marker CD28 deserves a mention. This membrane glycoprotein is a strong canditate for providing the hitherto mysterious costimulation, via ligation of B7, that is needed to prevent induction of anergy (Linsley et al 1991 ). One might have hoped that whatever molecule filled this role would also have explained the inability of T -cells in the thymus to make positive responses, in contrast to peripheral T -cells; alas, CD28 cannot do so, as it is well expressed by thymic T cells (Gross et al1992). The crucial molecular difference between thymic and peripheral T -cells that makes the former insensitive to costimulation must lie elsewhere. There remains a fourth possible mechanism, bystander suppression. The four regulatory subpopulations mentioned above, THO, THl, TH2 and mucosal T, each secrete at least one cytokine that can suppress the activity of other T -cells, from the range of IFNy, IL-4, IL-lO and TGFß (my colleagues A.K.Simon and J.S.Sieper find that IL-lO is not restricted to TH2 cells as has been thought, but is produced also by THl clones in man). The three-cell clusters that mediate T -T help (Mitchison 1990b) would provide the right kind of structure for these suppressive interactions to take place in, although that is only a speculation. Some sort of cytokine-mediated bystander suppression is frequently invoked as the mode of action of immune suppression genes (Mitchison 1991 ), but so far without much hard evidence. The best evidence so far of bystander suppression comes from the recent work on oral tolerance that is outlined below.
Oral tolerance is an old story with a new logic. As recently as two years ago, I was writing "some routes of administration proved more effective than others (for producing tolerance), for reasons that have never been entirely clear. For instance, oral administration is particularly effective, possibly because the liver can filter off particles before they reach lymphoid tissue." (Mitchison 1992). The portal filtration hypothesis traces back to the 1960's (Howard and Mitchison 1975), but has never found much experimental support. The new logic stems from two experimental findings, both concerning TGFß. One of these is that suppressor T cells generated by oral tolerization to myelin basic protein suppress in vitro and in vivo immune responses by the release of TGFß after antigen-specific triggering. Suppressor CD8 T cells from animals orally tolerized to myelin basic protein produce a suppressor factor upon stimulation with MBP in vitro that is specifically inhibited by anti- TGFß neutralising antibodies (Miller et al1992). In addition, active TGFß1 can be demonstrated in supernatants of these cells after stimulation. Originally described as a factor that causes anchorage-independent growth of fibroblasts, TGFß is a pleiotropic cytokine which mediates tissue repair (referred to by Mosmann .as an agent responsible for clearing up after the party IS over ). Of particular relevance is its ability to inhibit proliferation and differentiation of T cells (Wahl et al1988; Kehrl et al1987). The second important finding is that TGFß acts as an isotype-specific switch factor for IgA. Following the initial report that TGFß specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes (Coffman et al1989), a rash of confirmatory reports have appeared, some of which go into consIderable molecular detail concerning the mode of action of TGFß in the switch (e.g. lwasato et al1992). Taken together, these findings suggest that TGFß secreting T cells have the normal function of promoting local IgA production in the gut, where this class of immunoglobulin is mainly needed. They also exert an inhibitory effect on other T cells, and it is through this latter effect that oral tolerance operates. When activated through oral intake of an organ-specific antigen, presumably via uptake by M-cells into Peyer's patches, they migrate and localise preferentially in the organ, and can there exert a trigger-specific but effector-non-specific inhibitory effect on other T cells mediating auto-immune processes, as illustrated in figure 2. It is tempting to speculate on the possible importance of local tolerance induction in the gut in connection with the handling of dietary antigens, but that would take us far into realms of speculation. In the long run it is likely that attention will shift from oral administration of protein antigens to nasal administration of peptides. The gut is not the only site of secretion of IgA .across an epithelium. The lungs too are rich in IgAsecreting cells, and the airway offer a tempting alternative route for immune intervention, because of the relative simplicity of its pharmacokinetics. Peptides offer flexibility in the choice of epitope and greater scope for chemical manipulation than do intact proteins. David Wraith (Cambridge University, personal communication) is obtaining encouraging results in the suppression of EAE by nasally administered peptides. The question is entirely open whether Wraith's nasal route will turn out to be more effective than Gefter's subcutaneous one. Either way, negative regulation by peptides looks very promising indeed.
T -cell epitopes are now well understood as amino-acid nonamers binding to major histocompatibility complex molecules. Powerful methods have been developed for their identification through screening of recombinant and synthetic peptides. Multiple epitopes from a single protein are valuable for detecting T -cell reactivity in disease, currently in human immunodeficiency virus infection, and in the future in autoimmune disease. Surprises are likely to be encountered while exploring the T -cell repertoire in this way, such as positive as well as negative selection of self-reactivity. T-epitopes are likely to find important applications in therapy, particularly in down-regulation of the immune response. Multiple mechanisms of downregulation appear to operate, among which bystander suppression by TGFß-producing T -cells from the gut is of great current interest. Figure 1. 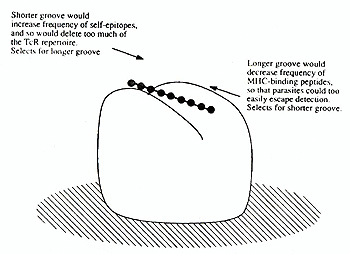 Evolutionary pressures on groove size in the MHC molecule
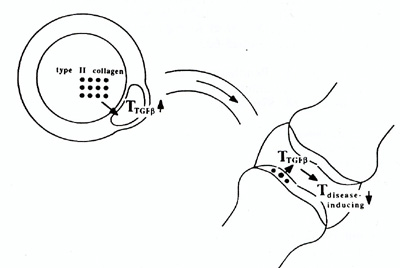
The presumed mechanism of oral tolerance. An organ-specific antigen, such as type II collagen, is taken up from the gut lumen by M-cells and passes into a Peyerrs patch, where it activates mucosal T cells specialised in TGFß production. These migrate through lymph ducts and blood into other parts of the body. When localised in an inflamed joint they are specifically stimulated by locally released type II collagen to secrete TGFß, which then non-specifically inhibits the growth and differentiation of the inflammation-inducing bystander T cells.
Adorini, L., Muller, S., Cardinaux, F., Lehmann, P.V., Falcioni,
F., Nagy, Z.A. 1988 In vivo competition between self peptides ansd
foreign antigens in T -cell activation. Nature 334:623-625 |