|
Deutsches Rheuma Forschungszentrum Berlin, Am Kleinen
Wannsee 5, W-I000 Berlin 39, FRG
Immunological diseases follow a characteristically fluctuating
course of relapse and remission, such as is illustrated in Fig.
1. This is true most obviously of diseases such as rheumatoid arthritis
which have a major autoimmune component, but it holds equally well
for chronic infectious diseases such as leprosy in which hypersensitivity
plays an important part. It is likely, but not definitely established,
that the fluctuations reflect an imperfect balance between opposing
forces within the immune system, and that these in turn reflect
the activity of opposing control genes. Among such control genes,
those of the major histocompatibility complex (MHC) are likely to
be the most important. Studies on the MHC and disease have tended
to focus on detrimental genes, that is, those which are positively
associated with the disease in question, predispose for it, and
presumably act as causal factors. Some of the autoimmune diseases
are tightly associated with particular HLA genes, such as is ankylosing
spondylitis (and certain forms of reactive arthritis) with HLA-B27.
For others the tightness of the association has become apparent
only as seemingly unrelated predisposing genes have been discovered
to share sequences in common. Thus an epitope shared between HLA-DRl
and HLADw4 explains well why both of these genes predispose for
rheumatoid arthritis [1]. The existence of a tight association suggests
that the disease process may be driven by presentation of a self-peptide
by the HLA molecule. Not only does this provide an attractively
simple picture of how the disease develops, but it also points the
way forward to new modes of treatment. From the tight associations
spring the present flurry of excitement concerning HLA-blocking
pep tides and monoclonal antibodies. In comparison, beneficial HLA
genes have suffered neglect. This seems a pity, if only because
it makes sense to try to understand what makes a patient get better.
The negative associations between HLA and disease seem on the whole
to be weaker than the positive ones, although this has not been
categorically estabiished. Another reason for neglect is that it
is less obvious how an MHC gene could inhibit an immune response,
in the way that these beneficial genes seem to do. One has to think
seriously about suppressive activity and suppressor cells, and those
are subjects that immunologists have learned to be cautious about.
Nevertheless they are the subject of this paper.

Fig.1. The disease pattern of relapse and remission,
characteristic of immunological diseases,
suggests that opposing activities operate within the immune system
Inhibitory MHC Genes
Examples of inhibition of immune responses by MHC genes are not
hard to find. Our recent survey lists some 20 in mouse and man [2].
What that listing did not include are the significant but only moderately
impressive negative associations between HLA and autoimmune disease
that have often been recorded, usually as by-products of surveys
aimed principally at verifying positive associations. Figure 2 gives
an example, showing the apparently beneficial effects of three HLA-DR
genes on rheumatoid arthritis in a recent study [3]. If I had to
choose just one example of a disease study, it would be the joint
work carried out by groups from Leyden and Caracas on HLA and lepromatous
leprosy [4]. Not only does this contain beautiful data, but also
it provides an amusing sidelight on paternity and family life in
Venezuela. Since then leprosy has become an arena for testing ideas
about genetic control of suscepbility to chronic infectious disease,
and more recent presentations of the topic are available [5]. Rather
than go over the whole list of inhibitory genes in this and other
areas again in detail, it seems more useful on the present occasion
to offer the following generalizations.
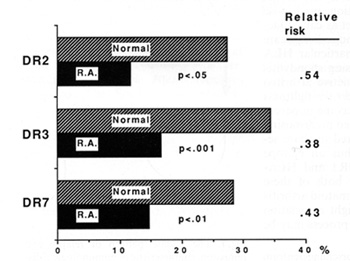
Fig.2. Negative associations between class II HLA genes
and rheumatoid arthritis, detected in a UK survey
1. As evidence of an immunoinhibitory effect, a negative association
between HLA and an autoimmune disease ( or other immunological disease,
such as allergy) is equivalent to a positive association with an
infectious disease.
2. Evidence of such associations often springs initially from population
surveys. Such data need eventually to be supported by the stronger
evidence that multiple-case family studies can provide. In the mouse,
studies on panels of recombinant inbred mice provide the equivalent
of family studies in man. It should be noted, however, that families
with multiple cases tend to have a high susceptibility background,
and that this will diminish the impact of protective genes (I am
grateful to H. 0. McDevitt for pointing this out to me).
3. As mentioned above, many immunological diseases have strong positive
HLA associations. This will tend to produce negative associations
for other HLA genes, and the more frequent such a gene is in the
study population, the more likely is this negative association to
reach significance.
4. Although a negative MHC association may be taken as prima facie
evidence of immunoinhibition in an immunological disease (and likewise
a positive one in an infectious disease), detailed immunological
study would be needed to substantiate the claim. This might involve
exploring the possibility of relieving the inhibition by in vitro
( or in the mouse by in vivo) treatment with anti-MHC monoclonal
antibodies, or other procedures.
5. The clear-cut inhibitory MHC genes have all so far turned out
to belong to class II. This is surprising, in view of the fact that
T cells able to mediate an inhibitory effect often have the CD8
phenotype. One can think of explanations, for instance, along the
lines of the phenomenon known in inbred mice where, when an active
H-2K allele (in a cytotoxic response) is replaced by an inactive
one, the previously inactive H 2D allele become active (and vice
versa). But the absence of class I genes still seems odd, and perhaps
further research will change the picture.
6. No MHC gene has been found to mediate inhibition exclusively.
All the genes which inhibit a response act positively in others
(this statement requires some qualification as regards HLA-DQ in
man, where most of the evidence for a positive effect comes from
in vitro studies with cloned T cells of "helper" phenotype).
7. Nevertheless a certain bias in the location of inhibitory activity
within the MHC is evident, both in mouse and man. This is respectively
towards H2 E and HLA -DQ .These genes are not, of course, homologous
in evolution, but they do share certain features in common. Both
seem to be secondary class II genes, with relatively few T cells
restricted by them, with relatively low expression, and with relatively
low polymorphism (none of these features are definitively documented,
unfortunately). Function seems to have flipped from one gene to
another during the evolutionary divergence of the two species.
One of the important issues of the day in this area is the claim
that the presence of asp-57 in HLA-DQ protects against insulin-dependent
diabetes. The most recent publication on this subject amounts to
a vigorous rejection of this claim on the basis of a segregation
study in multiplecase families [6], but the qualification about
this type of study, mentioned in point 2 above, means that judgement
should be suspended. I doubt if we have yet heard the last word
on this matter. Finally let me mention the recent study that raised
my interest in the present issue [7]. It showed that substitution
of H-2A b for H-2A k strongly inhibits the response of mice to F
liver antigen and does so more weakly for their response to Thyl
antigen. This provides a promising system for further study of mechanism.
Mechanisms of Inhibition
A comprehensive conceptual framework within which to consider mechanisms
of inhibition is much needed, and the main purpose of this paper
is to present one in the outline form shown in Fig. 3. In doing
so, I gladly acknowledge the benefit derived from discussions with
C. S. David and H. 0. McDevitt, and from the trenchant commentary
of Nepom [6]. This classification begins with a distinction between
intracellular and intercellular mechanisms, so that only in the
former does the inhibitory MHC product operate within the same cell
as the positive MHC (immune response, Ir) product. Intercellular
mechanisms corre

Fig.3. A classification of immunoinhibitory mechanisms
mediated by class II major histocompatibility complex molecules.
The inhibitory molecule or repertoire is shaded darker
spond roughly to what Nepom designates as "independent" inhibitory
activity, a category whose existence he questions. Intracellular
mechanisms further subdivide into intra- and intermolecular ones.
Intramolecular inhibition, for instance, might involve an amino
acid substitution in the :alfa 1 helix inhibiting the activity of
an Ir gene previously defined by substitution in the :alfa 2 helix.
This is a makeshift, as eventually the combination would be designated
simply as anew neutral allele. On the other hand, the category of
intracellular, intermolecular inhibitions has several examples,
mostly involving class I genes in the mouse. These I have discussed
some time ago [8]. The phenomenon of competition in the antiviral
cytotoxic response between H2K and H-2D mentioned above belongs
here, as also does the competition between heterozygous alleles
at H-2K or H2D that has been noted in the same type of response.
More relevant to our present discussion of class II inhibitory genes,
perhaps, is the competitive suppression that my group has studied
in the antiThyl response [9]. There the presence of an allogeneic
MHC molecule on a Thyl antigenic cell (but not on a neighboring
cell) can inhibit thc response. That this is truly a competitive
inhibition is strongly supported by our (unpublished) data de monstrating
loss of inhibition when the dose of antigen is increased. We do
not known for certain whether the allo-MHC antigen in this experiment
is presented by self-class II molecules in the usual way, but ifit
is then the gene(s) which encode them could be regarded as inhibitory
for the anti- Thyl response. In summary, the known instances of
intracellular, intermolecular inhibition boil down to antigenic
competition. The intercellular mechanisms subdivide into those that
result from negative or positive selection. In negative selection
the product of one MHC gene inhibits the activity of another by
deleting apart of its repertoire. The ikon for this category in
Fig. 2 depicts the T cell repertoire as subdivided into four parts
according to their restriction elements (e.g. H-2Aa, H-2Ab, H-2Ea,
H-2Eb); the part restricted by the inhibitory gene is shaded darker,
and there are holes in the other parts of the repertoire. This phenomenon
is now familiar in the context of superantigens, such as H-2E, or
the mls product. It has not yet been identified (to the best of
my knowledge) as a mechanism of inhibition resulting from class
II allelic substitution, although that may well be disclosed in
the future. Suppression mediated by positive selection is the most
challenging category, for this is our old friend the suppressor
T cell revived. In the formal sense used here, inhibition results
from positive selection when the product of one MHC class II gene
enables a group ofT cells to develop that are able to inhibit the
activity of another group of T cells that would otherwise perform
a response. The ikon shows the part of the repertoire that is restricted
by the inhibitory gene as biased towards inhibition, while the rest
of the repertoire is biased away from that activity (note the patches
of shading). As mentioned in the figure, current theory is that
inhibitory T cells could operate in alternative ways. One would
be, by engaging in inhibitory anti-idiotypic recognition, thus involving
Jerne's network. An alternative would be the secretion of inhibitory
or competitive lymphokines, such as do TH 1 and TH2 cells in the
mouse [10]. Other possibilities could be cited, such as antigen-specific
suppressor factors, but these seem too remote to be included in
the discussion. This is not the place to discuss these last alternatives
in detail. Inhibition via the network has a long history and much
experimental support (see my reviews [11,12]) and can be regarded
from various points of view. As K. Rajewsky has pointed out to me,
it could be no more than a form of mopping up, needed only to prevent
inescapable network interactions getting out of control. Or, as
I. R. Cohen proposes, self-macromolecules such as heatshock proteins
or myelin basic protein could induce a positive response within
the immune system, which would normally be contained by anti-idiotypic
suppression, but which would on occasion break out in the form of
autoimmune disease. As regards lymphokines, the evidence in the
mouse is convincing of mutual inhibition mediated by y-interferon
produced by TH 1 cells, and interleukin (IL)-4 and IL-10 produced
by TH2 cells. In man the position is less clear; perhaps atopy and
its control by therapeutic vaccination may offer the best example
of inhibitory T cell activity [13]. Now that we have this classification,
are we yet in a position to assign any of the known immunoinhibitory
effects to their correct slot within it? For the effects which matter
in human disease, of the type shown in Fig. 2, the answer is, not
yet. For mouse models some assignments can be made to the category
of positive selection and others to that of negative selection.
V -gene usage provides an important clue to the operation of negative
selection, and enhancement of the response by anti-class II antibody
does likewise for positive selection. But it must be emphasized
that assignments made on these bases are only provisional, because
it is always hard to exclude the possibility that some additional
mechanism is operating. This discussion has focussed on ways of
carving up CD4 class II-restricted T cells, the main regulatory
compartment of the immune system and arguably the single most numerous
and most important group of lymphocytes. They can be subdivided
according to restriction element, involvement in the network, lymphokine
secretion profile, and positive versus negative effect (and also
according to markers such as CD45R which discriminate between naive
and memory cells). Some of these are lineage markers while others
are not, and one needs to use ones wits when mixing the two [14].
It seems to me that the alignment of these various characteristics
is one of the most important items on the agenda of cellular immunology.
Why Are Immunoinhibitory Genes so Frequent?
It is hard to believe that autoimmune disease occurs with sufficient
frequency, or in a young enough age group, to have had much evolutionary
impact. As we have argued elsewhere [15], the driving force is more
likely to have been the hypersensitivity induced by chronic infection.
Most or all of the major tropical diseases are associated with hypersensitivity,
and nowhere is this more conspicuous than in leprosy. In that disease
immunopathological mechanisms are most threatening in borderline
cases. It is as though individuals at either end of the spectrum
are protected: at the tuberculoid pole (and in the much larger number
of individuals who are infected but never show clinical symptoms)
the immune system functions in its usual protective mode, while
at the lepromatous pole its protective functions are inhibited and
the parasite becomes free to multiply. Immunoinhibitory genes may
thus occur in human populations in the developed world largely as
a result of past selection for inhibition of infection-associated
hypersensitivity. The association noted above between immunoinhibitory
activity of MHC genes, low expression, and low polymorphism now
begins to make sense. The predominant activity of MHC genes is positive
where they function as immune response genes. Such genes are driven
to become intensely polymorphic, as a result of what the evolutionary
biologists have come to call "the Red Queen strategy." By this is
meant that anyone species lives within an environment provided by
other species, and as one evolves so must the others. The final
result is a great deal of evolutionary change but little real progress,
just as in Lewis Carrol's Through the Looking Glass where Alice
and the Red Queen hold hands and run, without getting anywhere.
Nowhere does this apply with greater force than in the coevolution
of immune response genes in the host and the antigen genes in parasites
to which they are opposed. This ceases to apply to MHC genes in
respect of their inhibitory function. In that case the interests
of the host and the parasite coincide; the Red Queen, so to speak,
comes out of play. We can therefore expect immunoinhibition to associate
with diminished polymorphism. The association with diminished expression
may occur because, on balance, such genes prove less valuable in
an evolutionary sense; they may even be on their way to total elimination
from the MHC gene pool. It is tempting to suppose that reduced class
II expression may provide a mechanistic signal for suppression within
the immune system, thus closing the evolutionary circle. All this
is of course highly speculative. The value of the evolutionary arguments
is that they focus attention on particular mechanisms, and also
that they identify the need for particular types of immunoepidemiological
data.
New Therapies: Combatting or Enhancing lmmunoinhibition
The proof of these ideas about inhibitory activity is whether they
lead on to new forms of therapy. In this context three lines of
current research look particularly promising. The first two concern
chronic infectious diseases in which immunoinhibitory activity has
long been suspected of preventing recovery, and where a novel form
of therapy offers hope of breaking through that barrier. The third
concerns the opposite problem, autoimmune disease in which the lack
of adequate immunoinhibitory activity may help cause the disease,
and where a novel form of therapy might rectify that defect. This
year a group from the Rockefeller University collaborating with
local researchers in Addis Ababa published their results on sublesional
administration of IL- 2 in leprosy [ 16] .This is the first trial
of lymphokine treatment in a chronic infectious disease, and it
used the lymphokine at something approaching physiological concentrations
(far less than has been used in cancer trials). The results were
encouraging, as judged by the histological response determined in
skin biopsies, and treatment with other lymphokines is planned.
From the point of view expression above, treatment of this sort
carries great promise as well as some hazard. If patients are to
be shifted along the spectrum towards the tuberculoid pole, it is
essential that they be moved out of the intermediate zone of hypersensitivity
and not into it; that will require careful patient selection. While
these results do not provide direct support for the TH 1TH2 concept,
they are at least compatible with it. Last year there appeared a
full report on the treatment of cutaneous leishmaniasis by immunotherapy
in the form of vaccination with bacille CalmetteGuerin CBCG) plus
killed leishmania organisms [17], a form of treatment that has also
been applied in leprosy. Results as good as those of conventional
chemotherapy were obtained. That reports includes a detailed and
thoughtful discussion of the possible mode of action; once again,
while many other possibilities remain open, an interpretation in
terms of competing Iymphokines seems attractive. The third attractive
line of research is gene therapy. After long debate and much hesitation,
we are about to witness the first gene therapy trials in man, starting
probably with cancer and with congenital enzyme deficiencies. A
strong case can be made for following these with trials in the hemoglobinopathies.
If all goes well, it would seem reasonable to consider such therapy
for cases of autoimmunity which have proved refractory to other
forms of treatment. The type of gene that one would wish to implant
are those shown in Fig. 2, or possibly an asp57 HLA-DQ if the doubts
mentioned above can be resolved. I am well aware of the difficulties:
how to protect an implanted allogeneic major transplantation antigen,
for example; and, for those genes which operate their inhibitory
effect through positive selection, how to obtain expression in thymic
epithelium. But with the technologies that are becoming available
these obstacles do not seem insuperable. Now many be the time to
start a serious research effort towards that goal.
References
1. Wordsworth BP, Lanchbury ISS, Sakkas LI, Welsh KI, Panayi GS,
Bell II (1989) HLA-DR4 subtype frequencies in rheumatoid arthritis
indicate that DRB I is the major susceptibility locus within the
HLA class II region. Proc Natl Acad Sci USA 86:10049-10053
2. Oliveira D GB, Mitchison NA (1989) Immune suppression genes.
Clin Exp Immuno175:167:177 3. IaraquemadaD,OllierW,AwadI, Young
A; Silman A, Roitt IM, Corbett M, Hay F, Cosh IA, Maini RN (1986)
HLA and rheumatoid arthritis: a combined analysis of 440 British
patients. Ann Rheum Dis 45:627-636
4. Van Eden W, Gonzalez NM, de Vries RR, Convit I, van Rood II (1985)
HLA-linked control of predisposition to lepromatous leprosy. I Infect
Dis 151:9-14
5. Li SG, de Vries RRP (1989) HLA-DQ molecules may be products of
an immune suppression gene responsible for Mycobacterium leprae
specific nonresponsiveness. Int I Leprosy 57:445-556
6. Nepom GT (1990) HLA and type 1 diabetes. Immunol Today 11 (9):314-315
7. Mitchison NA, Simon K (1990) Dominant reduced responsiveness
controlled by H2 (Kb)Ab. A new pattern evoked by Thy-1 antigen and
F liver antigen. Immunogenetics 32.104-109
8. Mitchison NA (1980) Regulation of the immune response to cell
surface antigens. In. Pernis B, Vogel HI (eds) Regulatory T lymphocytes.
Academic, New York, pp147-157
9. Lake P, Mitchison NA, Clark EA, Khorshidi M, Nakashima I, Bromberg
IS, Brunswick MR, Szensky T, Sainis KB, Sunshine GH, Favila-Castillo
L, Woody IN, Lebwohl D (1989) The regulation of antibody responses
to antigens of the cell surface: studies with Thy-1 and H-2 antigens.
In: Reif AE, Schlessinger M (eds) Cell surface antigen Thy-1. immunology,
neurology and therapeutic applications. Dekker, New York, pp 367-394
10. Mosmann TR, Coffman RL (1989) TH 1 and TH2 cells: different
patterns of Iymphokine secretion lead to different functional properties.
Annu Rev Immunol 7:145
11. Mitchison NA, Oliveira DBG (1986) Epirestriction and a specialised
subset of T helper cells are key factors in the regulation of T
suppressor cells. In: Cinader B, Miller RG (eds) Progress in immunology,
vol 6. Academic, London, pp 326-334
12. Mitchison NA (1989) Is genes in the mouse. In: Melchers F (ed)
Progress in immunology, vo] 7. Springer, Berlin Heidelberg New York,
pp 845-852
13. Plaut M (1990) Antigen-specific Iymphokine secretory patterns
in atopic disease. J ImmunoI144.4497-4500
14. Mitchison NA (1988) Suppressor activity as a composite property.
Scand J Immunol 28:271-276
15. Mitchison NA, Oliveira DBG (1986) Chronic infection as a major
force in the evolution of the supressor T cell system. Parasitol
Today 2:312-313
16. Kaplan G, Kiessling R, Tekelmariam S, Hancock G, Sheftel G,
Job CK, Converse P, Ottenhoff THM, Becx-Bleumenink M, Dietz M, Cohn
ZA (1990) The reconstitution of cell mediated immunity in the cutaneous
lesions of lepromatous leprosy by recombinant interleukin-2. J Exp
Med 169:893-908
17. Convit J, Castellanos PC, Rondon A, Pinardi ME, Ulrich M, Castes
M, Bloom B, Garcia L (1987) Immunotherapy versus chemotherapy in
localsed cutaneous leishmaniasis. Lancet 1 :401-405
|


