|
1 Shanghai Institute of Hematology, Shanghai Second
Medical University, Shanghai,People's Republic of China
2 Shanghai Zhong-Shan Hospital
3 Shanghai Children's Hospital, Shanghai, People's Republic of China
A. Introduction
Acute promyelocytic leukemia (APL) is considered to be a distinct
entity among the acute myeloid leukemias (AMLs). Hemorrhagic diathesis
often occurs and results in a rapid fatal outcome. The bleeding
episodes are usually attributed to thrombocytopenia and/or disseminated
intravascular coagulation (DIC), which is thought to result from
the release of a procoagulant factor from the promyelocyte granules
[1]. The use of daunorubicin in the induction therapy and improvement
in the supportive therapy has greatly raised the rate of complete
remission (CR) in APL [2, 3]. However, the increased mortality during
induction therapy is higher in APL than in other forms of AML [3,
4] and there are still cases which continue to be refractory to
induction chemotherapy. DIC remains a common lethal complication.
Induction of differentiation may be an alternative approach to the
treatment of APL. Retinoic acid (RA), an analog of vitamin A, is
one of the many agents which can induce differentiation and terminal
cell division of leukemic cells in vitro [5]. At the present time,
several cases of APL treated with 13-cis RA have been reported with
encouraging results [6-9]. In this paper, we report the in vitro
studies and therapeutic trials of 24 APL patients using all-trans
RA.
B. Materials and Methods
I. Patients
The diagnosis of APL was made according to the criteria of the French-American-British
(FAB) cooperative study group [10]. Every patient presenting to
our hospitals since early 1986 with a diagnosis of APL was included
in this stud y .The clinical characteristics of the 24 patients
with APL are shown in Table 1. There were 11 females and 13 males,
with a mean age of 35.5 years (range = 5-69 years). The total white
blood cell counts ranged from 0.5 x 109/liter to .8 x 109/liter,
including 20 cases (83.3%) with less than 3x10 high9/liter, 3 cases
(12.5%) with between 3 x 10 high9 and 10 x 10 high9/liter, and 1
case (4.1 %) with more than lOx 109/liter. The hemoglobin concentrations
ranged from 41 g/liter to 121 g/liter including 8 cases (33.3%)
wi th less than 60 g/li ter , 12 cases ( 50% ) between 60 and 90
g/liter and 4 cases (16.7%) with more than 90 g/liter. Platelet
counts ranged from 10 x 109/liter to 337 x 10 high9/liter including
15 cases (62.5%) with less than 50 x 10 high9/liter, 7 cases (29.2%)
between 50 x 109 and lOO x 10 high9/liter and 2 cases (8.3%) with
more than lOO x 109/liter. The percentage of promyelocytes in the
marrow ranged from 15.6% to 94%, with 22 patients having more than
30% and the remaining 2 patients having between 15.6% and 30%. Of
these 24 studied patients, 16 had never been treated. The other
eight (case # 1-# 8) had previously been treated with chemotherapy
[HOAP 1(1 H, Harringtonin (0.02-0.07 mg/kg/day); 0, oncovin (0.02-0.03
mg/kg/day); A, cytosine arabinoside ara -C); P, prednisone; C, cyclophosphamide)
, HOP, OH, COH, H]. Of the eight treated patients, three were in
relapse after 1- 30 months of CR and five were resistant to or could
not tolerate the chemotherapy (5-62 days of treatment). Twenty-two
of the patients showed mild to moderate hemorrhagic manifestations
(purpura, gingivorrhagia, gastrointestinal bleeding) but no laboratory
evidence of DIC prior to treatment with RA except for a positive
plasma protamin sulfate paracoagulation (3P+) in three of the cases.
II. Marrow Preparation and Culture
A modification of the method of Flynn et al. [6] for short-term
suspension culture was used. Marrow cells were aspirated from the
iliac crest, layered onto Ficoll-Hypaque (specific gravity 1.077),
and centrifuged at 800 g for 15 min. Interface cells were collected,
washed with McCoy's 5A medium, and resuspended at a concentration
of 5 x 10 high5 cells/mI in McCoy's 5A medium containing 15% fetal
calf serum. All-trans RA (Shanghai No.6 Pharmaceutical Factory,
Shanghai) was dissolved in absolute ethanol to a concentration of
1 mM and further diluted with the medium so that the final ethanol
concentration in the cultures was 0.1% and the final RA concentration
1µM. Controls were cultured in medium alone. (It had been previously
demonstrated that 0.1% ethanol had no effect on cell growth and
on differentiation of HL-60 cells [11].) All cultures were incubated
at 37° C in a 5% CO2 atmosphere for up to 7 days. Cell density was
determined by hemacytometer and cell viability by the trypan blue
dye exclusion method. Aliquots of cells were removed for morphological
examination on the 2nd, 4th, and 6th day of culture.

III. Morphological Studies
Differential counts were performed on cell smears stained with Wright's
solution. Chloroacetate esterase and alphanaphthyl acetate esterase
stains were performed using standard techniques [12]. Samples from
four cases were prepared for transmission electron microscopic study.
The nitroblue tetrazolium (NET) reduction assay was performed as
described by Francis et al. [13]. The percentage of cells containing
intracellular blue-black deposits was determined in 200 cells on
Wright's stained slide preparations.
IV. Colony Formation Assay
Blast cell colonies were grown as described by Minden et al. [14].
Conditioned medium was prepared from leukocytes (106 cells/mI) incubated
at 37° C for 7 days in McCoy's 5A medium with 10% fetal calf serum
and 1% (v/v) phytohemagglutinin-P (PHA-P) (DIFCO) and stored at
4° C until used. The preparation was termed PHA-LCM. Bone marrow
cells were plated at 1 x 10 high6 cells/ml using McCoy's 5A medium
supplemented with 0.3% agar, 20% fetal calf serum, and 25% (v/v)
PHA-LCM. GMCFU was determined according to the technique of Pike
and Robinson [15] for colony growth in agar. Briefly, 2 x 10 high5
marrow cells were plated in 35-mm tissue culture dishes over a feeder
layer of 1 x 10 high6 leukocytes from healthy donors. The plates
were incubated at 37° C in a humidified 5% CO2 atmosphere. L-CFU
colonies (more than 20 cells) were scored on day 8 and GM-CFU colonies
(more than 40 cells) on day 10.
V. Treatment of Patients
The 24 patients in this series received alltrans RA (45-100 mg/m²/day)
as the remission induction therapy. Informed consent was obtained
from all patients ( or their parents ). Peripheral blood counts,
bone marrow aspiration, and co agulation parameters (in 21 cases)
including thrombin time, prothrombin time, plasma protamin sulfate
paracoagulation test, euglobulin lysis test, and fibrinogen levels
were determined before the start of therapy and at regular intervals
thereafter. CR is defined as less than 5% blasts plus promyelocytes
in a normal cellular marrow with a normal peripheral blood count
and an absence of the signs and symptoms of leukemia on physical
examination [16]. Partial remission (PR) is defined as less than
5% blasts plus promyelocytes in a normal cellular marrow but with
a clinically moderate anemia. Blood transfusion and antibiotics
were given as supportive treatment when necessary.
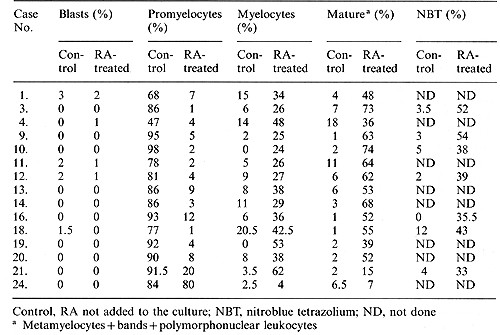
Table 2. Response of promyelocytes to
RA in suspension culture
VI. Continuation Therapy Following Complete Remission
Twenty-three patients were followed after attaining CR. Further
therapy was as follows: (1) Maintained by RA, 2030 mg/m²/day (six
cases), (2) maintained by RA, 20-30 mg/m²/day plus low-dose ara-C
(10 mg i.m. every 12 h) or low-dose Harringtonin (0.5 mg/m² i.v.
daily) in rotation (four cases), (3) maintained by low-dose ara-C,
10 mg i.m. every 12 h (five cases), (4) consolidated by chemotherapy
(HOAP) and maintained by 6mercaptopurine (2 mg/kg daily p.o.) and
methotrexate (10 mg/m², i.v. weekly), or cyclophosphamide (200 mg/m²,
i.v. weekly) (nine cases).

Fig. 1 A -D. Morphological maturation of leukemic cells
of case 10 in vitro and in vivo. A Cells cultured without RA, consisting
of promyelocytes with characteristic cytoplasmic granules, x 1000.
B Cells cultured with RA, showing maturation of granulocytes, x
1000. C Bone marrow before RA treatment. The predominance of promyelocytes
(76% ) indicates typical APL. D Bone marrow after 5 weeks of RA
treatment. Promyelocyte level under 2%, and restoration of normal
hemopoiesis without an aplasia phase are consistent with differentiation
induction
C. Results
I. In Vitro Studies
Leukemic bone marrow cells derived from 15 patients and incubated
for 7 days in suspension culture, with or without all-trans RA (1
µM), showed little change in cell density. Viability of both control
and RA-treated cells was consistently greater than 75%. Leukemic
promyelocytes from 14 patients showed morphologic and functional
maturation when cultured in the presence of RA (Table 2), (Fig.
1 A, B). The percentage of promyelocytes in the con trol group versus
the RA-treated group was 83.5% +-12.8% ² and 5.9% +-5.0% ² respectively.
The percentage of mature cells (metamyelocytes + bands + PMNs) was
4.7% +-4.5% ² and 53.9% +-15.4% ² respectively. The rate of NET
reduction in RA-treated cells was 42.0% +-7.5% ², significantly
higher than that of the control group (4.2% +-3.5%) ². 2 Results
represent the data from the patients studied and are expressed as
mean % +- standard deviation. To examine the progression of cellular
differentiation, we incubated cells from four patients with 1 µM
RA for various time intervals. After 48 h, morphologically recognizable
changes in the promyelocytes could be observed. The nu cleus became
larger and fewer primary granules were observed in the cytoplasm.
On the 4th day of culture, these cells gave rise to myelocytes which
contained specific, or secondaIy, granules. The nuclear chromatin
was more condensed and the nucleoli were either vague or no longer
visible. There was an elevated population of metamyelocytes which
had indented or horseshoe-shaped nuclei and cytoplasm filled with
both primary and secondary granules appearing by day 6, as well
as some band and fully mature granulocytes. When the cultures were
continued for 7- 8 days, the relative number of mature granulocytes
increased. Cytochemical analysis showed that in the control cells
chloroacetate esterase activity varied from mildly to moderately
positive, while in the RA-treated cultures intensely positive granules
were seen, either diffusely scattered or accumulated in some portion
of the cytoplasm. The majority of control cells showed weak nonspecific
esterase activity while RA-treated cells had a stronger reaction.
Transmission electron microscopic examination of four cultures confirmed
that in the presence of RA the cells had been differentiated to
mature granulocytes. Condensation of the heterochromatin became
evident and the nucleus had often been changed to a bean-shaped
or even a segmented form. Neutrophilic granules were smaller and
diffusely scattered throughout the cytoplasm. Azurophilic granules
were markedly decreased.
II. Clinical Studies
Twenty-four patients were treated with all-trans RA as a single
agent. All achieved both PR and CR except the one patient ( # 24)
whose cells were not inducible when cultured with RA in vitro. Subsequent
bone marrow examination of this patient revealed a continuing proliferation
of leukemic promyelocytes. When ara-C (10 mg) was added intramuscularly
every 12 h, the patient achieved CR in 98 days (Table 1). In the
12 patients studied who responded to the induction differentiation
effect of RA, L-CFU growth was predominant (163.3+- 129.0 colonies)
and GM-CFU suppressed (0.63+- 1.3 colonies) prior to treatment.
GM-CFU reached normal levels (100.2+- 55.1 colonies) with little
or no growth of LCFU after CR was achieved.
III. Pattern of Clinical Response to trans- Retinoic Acid
Systematic observation of the peripheral blood counts during RA
treatment of the previously untreated patients revealed some specific
patterns of change. There was a progressive rise in the total white
blood cell count which started with ini tiation of treatment and
which reached a peak between 7 and 14 days. After this, the white
blood cell count fell with the progressive maturation of granulocytes.
Increase in platelets was most prominent after 3 weeks. Elevation
of the hemoglobin concentration appeared reluctant and slow. Bone
marrow aspirate revealed that hypercellularity existed throughout
the RA treatment. Partial remission could be expected within 1 month
(Fig. 1 C, D). Therapy with oral all-trans RA was accompanied by
mild toxicity that consisted of dryness of the lips and skin (100%),
headache (25%), nausea or vomiting (20.8%), moderate bone or joint
pain (12.5%), and mild exfoliation (8.3%). Two patients had elevated
SGPT. All of these side effects were well tolerated or alleviated
when the dosage of oral RA was reduced.
IV. Disseminated Intravascular Coagulation
Coagulation parameters, including thrombin time, prothrombin time,
plasma protamin sulfate paracoagulation test (3P), euglobulin lysis
test, and fibrinogen levels, were measured simultaneously, in 21
patients, at the beginning of RA therapy and throughout the course
of treatment. Of these patients, 18 who were normal in coagulation
parameters prior to the start of RA therapy showed no changes during
treatment. The other three patients who had been previously treated
and who were 3P( + ) became negative 7-10 days after RA. Therefore,
DIC or other hemorrhagic complications did not occur when patients
with APL were induced to remission with RA.
V. Duration of Clinical Remission
Twenty-three patients were followed after induction of CR (Table
1 ). Of the six patients maintained on RA alone, four were still
in remission for a period of 5 10 months. Two patients relapsed
in 2 and 4 months. Among the four patients maintained on RA with
either low-dose ara-C or low-dose Harringtonin in rotation, three
relapsed within a period of 4- 5 months. Of the five patients maintained
on low-dose ara-C alone, one case ( * 2) was lost to follow-up,
one relapsed in 4 months, and the other three remained in CR for
1 + to 5 + months. Of the remaining nine patients who were consolidated
by chemotherapeutic regimens and maintained on 6-mercaptopurine,
methotrexate, or cyclophosphamide, two relapsed and seven have been
in CR from 1 + to 8 + months. The new population of APL promyelocytes
at relapse differed morphologically from those present at the start
of treatment and were resistant to all-trans RA induction of differentiation
in vitro.
D. Discussion
Recent approaches in treatment of leukemia include the use of "differentiation-inducing
agents" such as RA, vitamin D3, or low-dose ara-C [17-19]. Numerous
studies both in vitro and in vivo have revealed that RA is a potent
inducer of myeloid differentiation, both in the promyelocytic cell
line HL-60 as well as in fresh promyelocytes from patients with
APL, and at a concentration that was pharmacologically obtainable
in man [11,20]. 13-cis RA and all-trans RA were equally effective
in induction of dif ferentiation in vitro [5]. Our studies confirm
that in vitro leukemic promyelocytes could be induced by all-trans
RA to differentiate toward mature granulocytes. One exception was
that the cells from patient * 24 were resistant to RA induction.
The morphological characteristics of these RA-resistant cells revealed
a scanty cytoplasm with less-prominent coarse granulation. The differences
in sensitivity to RA may be due to the heterogeneous entities of
APL [21, 22]. In 1983, Flynn et al. [6] described the first case
of APL treated with 13-cis- RA. Unfortunately, this patient died
from disseminated candidiasis although there was a marked elevation
in his peripheral granulocyte count after 2 weeks of treatment.
Nilsson [7] reported a 30-year-old woman with APL in relapse for
10 months; she was treated by 13-ci,s RA (1 mg/kg) and began to
respond after 1 month, and normal blood and bone marrow pictures
continued for 11 months. Daenen et al. [8] reported a 33-year-old
patient with refractory APL complicated by fibrinolysis and aspergillus
pneumonia. He was treated with 13-cis RA (80 mg/day) alone, and
attained a CR after 7 weeks. Recently, Fontana et al. [9] reported
one case of refractory APL treated with 13-ci,s RA (lOO mg/m²) which
resulted in CR after 13 days. In vitro studies of this patient's
leukemic blasts showed differentiation in the presence of RA. Sampi
et al. [23] reported a 58-year-old Japanese man who also had relapsed
APL and failed to respond to etretinate (a form of retinoid) and
dactinomycin, although the leukemic cells were sensitive to all-
tran,s RA ( 10 -6 10- 7 M) in vitro. We have treated our patients
with all-trans RA and found that all-trans RA was not only effective
in patients who had been refractory to chemotherapy, but also effective
in those with "de novo" APL. Moreover, we were able to find predictive
value in the in vitro differentiation studies. The single patient
who was resistant to RA induction failed to show marrow improvement
when treated with RA as the sole agent. According to most authors,
the main disquieting problem of APL is death during induction treatment
[3, 4, 24], especially because of intracerebral hemorrhage. DIC
is the most common complication of APL. Its severity and frequency
are often aggravated by chemotherapy despite the use of heparin.
In this study we report no aggravation of hemorrhagic manifestation
or appearance of coagulation parameter abnormalities suggesting
DIC during the course of RA treatment. This would be one of the
striking advantages over aggressive chemotherapy which could destroy
the leukemic cells and cause the release of procoagulant factors
from the azurophilic granules into the circulation. It is possible
that the leukemic cells are not destroyed during treatment ofAPL
with RA, but that they have differentiated, undergone terminal cell
division, and lost the capacity to release these coagulant factors
during this process. The fact that there was no decrease, but rather
an increase, of marrow cellularity during induction therapy supports
this possibility. The role of all-trans RA in the maintenance of
remission is undetermined. Two cases of APL, reported by Daenen
et al. [8] and Fontana et al. [9], relapsed in 6 and 12 months respectively.
In our series, the patients were further treated with four different
regimens after CR was induced, but it is too early to conclude which
of these is the most effective. From the data obtained from both
our clinical survey and the cytogenetic studies showing the persistence
of abnormal clones (unpublished data), we suggest that intensive
chemotherapy after CR may be beneficial. The knowledge about the
side effects of oral RA is mainly from the dermatological literature.
Our data are compatible with other reports on the toxicity of oral
all-trans RA [25]. The toxicity of 13cis RA has been shown to be
relatively lower than all-trans RA [25], but in our experience the
side effects were well tolerated by the patients, some of whom have
been taking RA for more than 10 months with no severe untoward effects.
Based on these observations, we conclude that all-trans RA is an
effective agent for obtaining CR in APL. How to maintain and prolong
the duration of the CR, however, requires further study.
Acknowledgments.
We are grateful to Professor Samuel Waxman of Mount Sinai School
of Medicine for his kind comments and suggestions in editing this
manuscript, and to other physicians and hematologists at Shanghai
Rui-Jin Hospital, Shanghai Zhong-Shan Hospital, Shanghai Chang-Zhen
Hospital, and Shanghai Institute of Pediatrics for providing samples
and care of patients studied. We are also grateful to Zhao Jin-chai
for expert technical assistance.
References
1. Jones ME, Saleem A (1978) Acute promyelocytic leukemia: a review
of literature. Am J Med 65: 673-677
2. Bernard J, Weil M, Boiron M, Jacquillat C, Gemon MF (1973) Acute
promyelocytic leukemia: results of treatment by Daunorubicin. Blood
41: 489 -496
3. Drapkin RL, Timothy SG, Dowling MD, Arlin Z, Mckenzie S, Kempin
S, Clarkson B (1978) Prohylactic heparin therapy in acute promyelocytic
leukemia. Cancer 41:2484-2490
4. Cordonnier C, Vernant JP, Brun B, Heilmann MG, Kuentz M, Bierling
P, Farcet JP, Rodet M, Duedari N, Imbert M, Jouault H, Mannoni P,
Reyes F, Dreyfus B, Rochant H (1985) Acute promyelocytic leukemia
in 57 previously untreated patients. Cancer 55: 18- 25
5. Koeffler HP (1983) Induction of differentiation of human acute
myelogenous leukemia cells: therapeutic implications. Blood 62:
709- 721
6. Flynn P, Miller W, Weisdorf D, Arthur D, Banning R, Branda R
(1983) Retinoic acid treatment of acute promyelocytic leukemia:
in vitro and in vivo observations. Blood 62: 1211-1217
7. Nilsson B (1984) Probable in vivo induction of differentiation
by retinoic acid of promyelocytes in acute promyelocytic leukemia.
Br J Haematol 57: 365-371
8. Daenen S, Vellenga E, Van Dobbenbugh OA, Halie MR (1986) Retinoic
acid as antileukemic therapy in a patient with acute promyelocytic
leukemia and aspergillus pneumonia. Blood 67: 559-561
9. Fontana JA, Roger JS, Durham JP (1986) The role of 13-cis retinoic
acid in the remission induction of a patient with acute promyelocytic
leukemia. Cancer 57: 209-217
10. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG, Gralnick
HR, Sultan C (1976) Proposals for the classification of the acute
leukemia. Br J Haematol 33: 451-458
11. Breitman TR, Selonick SE, Collins SJ (1980) Induction of differentiation
of the human promyelocytic leukemia cell line (HL-60) by retinoic
acid. Proc Natl Acad Sci USA 77: 2936-2940
12. Yam LT, Li CY, Crosby WH (1971) Cytochemical identification
of monocytes and granulocytes. Am J Clin Pathol 55: 283 290
13. Francis GE, Guimaraes JETE, Berney 11, Wing MA (1985) Synergistic
interaction between differentiation inducers and DNA synthesis inhibitors:
a new approach to differentiation induction in myelodysplasia and
acute myeloid leukemia. Leuk Res 9: 573581
14. Minden MD, Buick RN, McCulloch EA ( 1979) Separation of blast
cell and T lymphocyte progenitors in the blood of patients with
acute myeloblastic leukemia. Blood 54: 186-195
15. Pike BL, Robinson WR (1970) Human bone marrow culture in agar
gel. 1 Cell Physiol 76: 77 -84
16. Vogler WR (1985) Post-remission therapy for acute myelogenous
leukemia. In: Bloomfield CD (ed) Chronic and acute leukemias in
adults. Martinus Nijhoff, Boston, p 209-228
17. Gold El, Mettelsmann RH, Itri LM, Gee T, Arlin Z, Kempin S,
Clarkson B, Moore MAS (1983) Phase I clinical trial of 13-cis retinoic
acid in myelodyplastic syndromes. Cancer Treat Rep 67: 981-986
18. Koeffler HP, Hirji K, Itri L (1985) 1,25Dihydroxyvitamin D3:
in vitro and in vivo effects on human preleukemic and leukemic cells.
Cancer Treat Rep 69: 1399 1407
19. Degos L, Castaigne S, Tilly H, Sigaux F, Daniel MT (1985) Treatment
of leukemia with low-dose Ara-C: a study of 160 cases. Semin Oncol
12: 196 199 [Suppl 3]
20. Breitman TR, Collins SJ, Keene BR (1981) Terminal differentiation
of human promyelocytic leukemic cells in primary culture in response
to retinoic acid. Blood 57: 1000-1004
21. Golomb HM, Rowley 1D, Vardiman 1W, Testa 1R, Butler A (1980)
"Microgranular" acute promyelocytic leukemia: a distinct clinical,
ultrastructural, and cytogenetic entity. Blood 55: 253- 259
22. Tomonaga M, Yoshida Y, Tagawa M, 1innai I, Kuriyama K, Amenomori
T, Yoshioka A, Matsuo T, Nonaka H, Ichimaru M (1985) Cytochemistry
of acute promyelocy tic leukemia (M 3): leukemic promyelocytes exhibit
heterogeneous patterns in cellular differentiation. Blood 66: 350-357
23. Sampi K, Honam Y, Hozumi M, Sakurai M (1985) Discrepancy between
in vitro and in vivo induction of differentiation by retinoids of
human acute promyelocytic leukemia cells in relapse. Leuk Res 9:
1475- 1478
24. Ruggero D, Baccarani M, Guarini A ( 1977) Acute promyelocytic
leukemia: results or therapy and analysis of 13 cases. Acta Haematol
(Basel) 58:108-119
25. Windhorst DB, Nigra T (1982) General clinical toxicology of
oral retinoids. J Am Acad Dermatol 6: 675-682
|


