|
Institute for Immunology and Genetics. Ger man
Cancer Research Centre. Im Neuenheimer Feld 280, 6900 Heidelberg, The thymus represents a crucial phase in the differentiation of
T cells, from their earliest precursor committed to the T-cell lineage
in the bone marrow, to the full array of peripheral T-effector cells.
Specification of T-cell subsets, generation of receptor diversity.
selection of self-MHC-restricted T -cell precursors, and induction
of selftolerance are thought to be largely or exclusively intrathymic
events [24]. How such complex functional events relate to the relatively
simple structure of the thymus is poorly understood. It has become
apparent that the pattern ofT-cell reactivity is selected by the
environment [6, 26] in which T cells develop rather than being strictly
genetically fixed; thus interest has focused on the definition of
such selection sites. To this end direct cell-cell interactions
between cells of the T -cell lineage and nonlymphoid stromal cells
in the murine thymus have been characterized [25, 13. 14]. These
interactions preexist in vivo, can be isolated as intact multicellular
lymphostromal complexes by differential digestion of the thymus.
and are thus amenable to analysis in vitro. At least three lymphostromal-cell
interactions can be discerned: ( I) between T cells and macrophages
(MØ), (2) between T cells and dendritic cells (DC) (both
I and 2 referred to as thymocyte rosettes, T-ROS). and (3) between
T cells and epithelial cells (thymic nurse cells, TNC). T -ROS and
TNC are obtained as sequential fractions during digestion, thereby
making possible a separate isolation of distinct complex types.
A comparison between these interaction structures revealed the following
salient points [25, 13-16. 7]. All three interactions seem to be
obligatory steps for T -cell differentiation; their frequency correlates
with ontogeny of T -cell maturation and is unaffected by the immune
status of the animals. T -cell-stromal-cell recognition in vivo
does not require syngeneity but occurs between fully allogeneic
partner cells [16]. The T-cell subsets engaged in stromal-cell interactions
(2%-3% ofall thymocytes) are immature in surface antigen phenotype
and enriched in cycling cells over unselected thymocytes. When the
entry of donor bone marrow (BM)-derived Thy 1.I pre- T cells was
followed in the thymus of congenic Thy 1.2 hosts. they were found
to interact first with macrophages. second with epithelial cells.
and third with dendritic cells. indicating a temporal hierarchy
of Iymphostromal recognition during T-cell development. These kinetics
do not necessarily imply a coli near maturation sequence since precursor
product relationships are not known. By direct comparison of the
appearance of ddonor T cells in Iymphostromal-cell complexes after
isolation in vitro, with the concomitant localization of donor T
cells in situ, M ØROS and TNC were located to the cortex
and DC-ROS to the medulla (Kyewski. unpublished data). Though the
recognition structures governing these interactions are not known.
it is surmised from indirect evidence in radiation chimeras that
self-MHC determinants at least in part specify these interactions
[6, 26]. Given the observation that both cortical epithelial cells
and medullary dendritic cells express high amounts of class II MHC-antigens
constitutively in vivo (whereas cortical macrophages were found
to be l-A/E negative), we tested whether non-MHC-antigens may havc
access to the thymus and be presented to maturing thymocytes during
their maturation in vivo. This question bears particular relevance
to the problem of where developing T cells expressing antigen-specific
receptors are first confronted with nonMHC-self-antigens and where
self-tolerance takes place. Recent evidence indicating that tolerance
induction is MHC restricted would favor T -cell-accessory-cell interactions
at such sites [8, 18, 19]. 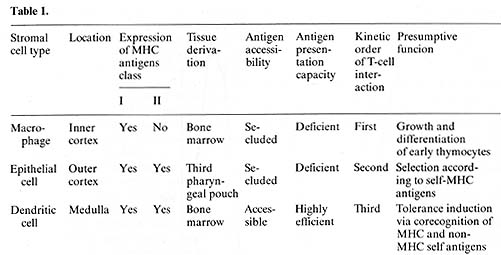
Intrathymic antigen presentation was assayed by coculture of antigen-specific
l-Arestricted cloned T -helper cells with purified irradiated thymic
Iymphostromal-cell complexes [II, 15]. As antigens we used myoglobin,
L-glutamic acid high 60-L-alanine high 30 L-tyrosine high 10 (GAT),
and keyhole limpet hemocyanin (KLH). Proliferation of T cells was
measured 72 h after culture in vitro by (³H]thymidine uptake. Antigen
was either injected intravascularly (i.v.) before isolation of the
stimulator population or added to the culture in vitro. After injection
of 0.5 mg myoglobin/g body weight i.v. in to C57BL/Ka mice, T-ROS
copurified with specific stimulation of l-A b-restricted myo globin-specific
T-helper cells. This antigenspecific stimulation was a property
of Thy1.2-negative stromal cells (anti- Thy 1.2 antibody plus complement
treatment did not alter the presentation capacity of T-ROS) and
could be inhibited by more than 90% after pretreatment of the stimulator
population with anti l-Ab monoclonal antibody and complement. Antigen
traffic to the thymus in vivo was dose dependent within the range
of 1.0-0.25 mg m yoglobin/g body weight. Threshold doses for thymic
and splenic antigen-presenting cells (A PC) required to present
antigen were similar, indicating no significant seclusion in vivo
of A PC enclosed in the T-ROS fraction. Similar results were obtained
after injection of KLH (molecular weight 3 x10 high 6) and GAT (molecular
weight, I x10 high 5) [ 15]. When kinetics of antigen persistence
in the thymus were measured, antigen-specific stimulation of T cells
was demonstrable up to 48 h after injection i.v. (Fig. IA). The
prolonged presence of antigen within the thymus argues against a
trivial explanation of these resuIts, namely the uptake of antigen
by stromal cells (which were secluded in the intact organ), after
disruption of the tissue context during the isolation procedure.
Although myoglobin (molecular weight 17000) is rapidly cleared from
the circulation, the APC activity of T -ROS was unchanged when tested
15 min or 12 h after injection of antigen.  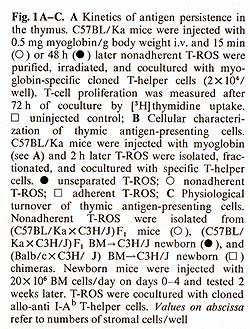 In further defining the cell type(s) responsible for uptake of antigen in vivo and presentation in vitro we separated T-ROS stromal cells into adherent and nonadherent fractions. More than 90% of adherent stromal cells are composed of I-A/ E-negative, F 4/80-positive, phagocytic MØlike cells, whereas the nonadherent stromal cells contain 50%-80% nonphagocy tic F 4/80-negative, I-A/E-positive DClike cells [I, 14]. When separately tested for APC activity after injection of myoglobin i.v., the nonadherent fraction contained all functional APC, whereas the adherent-cell fraction even when pulsed with additional antigen in vitro remained nonstimulatory (Fig. 1 B). Though this separation method needs further confirmation by enrichment protocols using strictly lineage-specific surface markers. it was reproducibly found that depletion of strongly adherent MØ did not affect the ability of the T -ROS fraction to present antigen. The lack of class I I MHC-antigen expression on cortical macrophages forming T -ROS is compatible with this result. In order to test DCs and TNCs separately for their accessibility and capacity to present antigen, we turned our attention to their different embryonic origins. DCs are strictly bone marrow derived whereas epithelial cells are derived from the third pharyngeal pouch. Thus, PI> (P1 X P2) F1 radiation chimeras were analyzed in which DCs were com pletely replaced by P1-type cells and epithelial cells remained of the F1 type. When antigen presentation by cells isolated from such animals was tested 10 weeks after reconstitution with T -helper cells restricted to P2type I-A antigens, no T -cell proliferation was measured using either purified T -ROS (that is bone-marrow-derived stromal cells) or epithelial cells as stimulators. The latter, however, expressed class II-MHC-determinants of P2-type abundantly, as determined by fluorescence microscopy. This lack of antigen presentation by thymic epithelial cells after injection of myoglobin i.v. could not be overcome by providing optimal doses of antigen in vitro [15]. This result indicates an intrinsic deficiency of epithelial cells in stimulation of T-helper cells, rather than a seclusion from antigen in vivo. It is not clear to date whether the epithelial cells lack the ability to process antigen and/ or fail to produce obligatory costimulation factors (e.g., interleukin-I). Interestingly, thymic epithelial cells have been successfully grafted across allogeneic barriers without being rejected [21]. Given the assumption that DCs are responsible for antigen presentation in the thymus, we further assessed the physiological turnover of thymic DCs (equivalent here to nonadherent T-ROS). To this end we used nonradiation chimeras. Newborn P1 mice were given multiple injections with F1 bone marrow cells at daily intervals. Such animals establish a stable bone marrow chimerism which is proportional to the dose of donor cells injected (Kyewski, unpublished data). In such "normal" hosts, without prior ablation of bone marrow-derived hemopoietic lineages, F1-BM-derived cells establish stem-cell chimerism and replace host cells during physiological turnover. When nonadherent T-ROS from such F1> P1 newborn chimeras were cocultured with cloned T -helper cells restricted to P2-type I-A antigens, specific proliferation was detected (Fig. I C). This proliferative response amounted to about 10%-20% of the magnitude induced by normal F1 mice-derived T -ROS, indicating a significant replacement in the thymus of host-type DCs by cells of donor origin. The result indicates that medullary DCs, in contrast to cortical epithelial cells, undergo a constant physiological turnover and replacement by extrathymic DCs. Thus, in addition to the direct entry of blood-bome antigens into the thymus, circulating antigen-Iaden-DCs may contribute to the spectrum of intrathymically presented antigens. The described results, in concert with earlier studies on these cell interactions [25, 13-16, 7], indicate a strict compartmentalization of thymic stromal cells with regard to their accessibility to circulating antigens and their intrinsic capacity to present these antigens to T cells. Macrophages and epithelial cells (here isolated by virtue of their interactions with thymocytes in vivo) seem to be highly inefficient in presentation of soluble protein antigens and are presumably secluded from blood-borne antigens by avascular blood-cortex barrier. In contrast, presenting DCs are strictly confined to the medulla, which in turn displays a vascular architecture permissive to the passage of macromolecules [20]. Thus, an important aspect of cortex/medulla dichotomy with regard to T -cell recognition resides in either the prevention or facilitation of T-cell encounters with non-MHC antigens in conjunction with self MHC-antigens. In the following we speculate on the possible roles of the three recognition steps in the context of the development of the T -cell repertoire. Pre- T cells probably enter the thymus at the cortical side of the corticalmedullary junction and first interact with and proliferate around macrophages in the inner cortex (Fig.2). 
1. Austyn JM, Gordon S (1981) F4/80, a mono clonal antibody directed
specifically against the mouse macrophage. Eur J Immunol 10: 805-811
2. Een-Ishay Z, Yoffey JM ( 1972) Ultrastructural studies of erythroblastic
islands of rat bone marrow. II Resumption of erythropoiesis in erythropoietically
depressed rebound marrow. Lab Invest 26:637-647 |