R. Mertelsmann 2, and M. A. S. Moore 2 Hämatol. Bluttransf. Vol 29 |
|
Supported by NCI grant CA 32516, ACS grant CH3G,
grant P110311-1 from the Deutsche Forschungsgemeinschaft, and the
Gar Reichman Foundation
As described elsewhere in this volume by Welte et al., a human pluripotent hemopoietic colony-stimulating factor (CSF) was purified to apparent homogeneity from media conditioned by the human bladder carcinoma cell line, 5637. The puritied material supports colony formation in vitro from human multipotential (CFUGEMM), early erythroid (BFU-E), granulocyte and monocyte (CFU-G, M) progenitor cells4. A murine CSF, lnterleukin 3 or multi-CSF, with similar activities on normal mouse bone marrow [1,2], has recently been purified [3] and genetically cloned [4]. lnterleukin 3 was originally detected by its ability to induce 20-alpha-OH-steroid dehydrogenase (20alfaSDH) in cultured spleen cells of nu/nu mice [5]. Later on it was discovered to have biological activities on a wide range of hemopoietic cells: progenitor cells of erythrocytes, megakaryocytes, granulocytes, monocytes and eosinophils, mast cells and their precursors, and possibly lymphocytes [2, 6-8]. Since come activities of lnterleukin 3 and pluripotent CSF, or Pluripoietin, on hemopoietic progenitor cells appeared similar, we screened for additional biological effects of Pluripoietin.
Normal human bone marrow cells taken from volunteers after informed consent were separated by density gradient centrifugation on Ficoll, adherence to plastic surfaces and depletion of cells rosetting at 4 °C with neuraminidase-treated sheep red blood cells. When this cell population was cultured in methylcellulose as described [9], Pluripoietin in the absence of phytohemagglutinin-stimulated lymphocyteconditioned medium (PHA-LCM) supported colony formation from CFU-GEMM and BFU-E, suggesting that pluripotent CSF acts directly on early progenitor cells, not via macrophages or T -lymphocytes as accessory cells of hematopoiesis. In agar cultures [10], Pluripoietin induced mostly neutrophil colonies by day 7, and neutrophil, macrophage, and mixed neutrophillmacrophage colonies as well as some eosinophil clusters by day 14. Furthermore, Pluripoietin induced the development of immature precursors of colonyforming progenitor cells of granulocytes and macrophages [ II ]. This was studied by incubating low-density, nonadherent, and T -cell-depleted marrow cells in liquid culture in the presence of Pluripoietin for 7 days prior to agar culture, in a granulocytepotentiating activity (DeltaGPA)-type [12] or precursor of CFU-G, M progenitor cell (pre-CFU-c) [13] assay. There are no reports of I nterleukin 3 tested in such assays, but it appears to have a similar effect on murine stem cells in vitro prior to trans plantation [14].
Normal human peripheral blood monocytes were isolated by adherence procedures as described [15]. When cultured in the presence of Pluripoietin from day I through 4, monocytes/macrophages showed marked spreading and an increase of adherent cell protein, suggesting increased protein synthesis as compared with untreated controls [ 11 ]. This effect was not seen when Pluripoietin was added at day 4 of culture or later, possibly because macrophages produce their own CSF. Pluripoietin did not increase the production of H2O2-producing enzymes or anti-Toxoplasma activity in macrophages when added after 3 days of culture [15]. Interleukin 3 was not reported to be active on macrophages, but its activity in supporting long-term growth in vitro of natural cytotoxic effector cells [ 16] and histamine-producing cells [6] may reflect activities on mature hemopoietic cells.
Differentiation of leukemic cell lines in vitro can be achieved by a variety of nonphysiologic [ e.g., dimethylsulfoxide (DMSO), phorboldiesters] and physiologic ( e.g., retinoic acid, vitamin D3) inducers [17]. Murine granulocyte-CSF (G-CSF) is known to be a potent inducer of differentiation of WEHI-3B (D + ) murine myelomonocytic leukemia cells, whereas Interleukin 3 lacks this activity (for review, see [ I ]). We tested Pluripoietin for leukemia-differentiating activity (GM-DF, leukemia-differentiating activity for granulocyte and macrophage pathway) in a clonal assay system described by Metcalf [18], using murine WEHI-3B (D+) and human HL-60 promyelocytic leukemia cell lines [11]. Quantitation of GM-DF was obtained by incubation of leukemic cells in agar with serial dilutions of pluripotent CSF. Pluripoietin had GM-OF activity on both cell lines. However, HL-60 required approximately fivefold higher concentrations of Pluripoietin to achieve 50% differentiated, spreading colonies versus undifferentiated tight blast-cell colonies than did WEHI-3B (D+) [II]. The human leukemia cell line KG I (courtesy Dr. H. P. Koeffler) responded to Pluripoietin with increased colony formation in agar and increased (³H] thymidine incorporation after 24-48 h in suspension culture. This might indicate that the GM-OF activity of Pluripoietin reflects the differentiating capacity of leukemia cell lines rather than an intrinsic property of the factor. Table 1. Murine mast-cell growth
factor activity of Pluripoietin 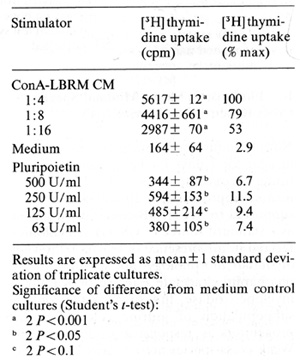 Table 2. Biological activities of purified human Pluripoietin and murine Interleukin 3 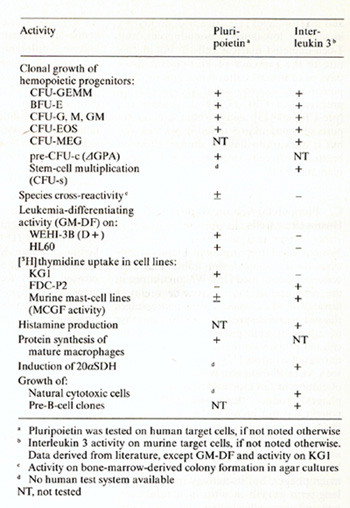
E. Pluripoietin Shows Minimal Species Cross-Reactivity on Murine Cells Normal mouse bone marrow cells cultured in agar for 7 days in the presence of saturating concentrations of Pluripoietin formed approximately ]0% of the colonies supported by WEHI-3B-conditioned media as a standard source of CSF(s). All colonies formed in the presence of Pluripoietin were of similar morphology, not staining for alpha-naphthyl-acetate esterase or Kaplow's myeloperoxidase; this suggests that only a subpopulation of murine colony-forming progenitors is responsive to Pluripoietin. Weak cross-species activity was also found on continuous murine mast-cell lines, established as described from murine longterm bone marrow cultures [ 19]. Five thousand cells per well of a mast-cell growth factor (MCGF)-dependent murine mastcellline were incubated for 24 h at 37 °C in 96 well plates with serial dilutions of growth factors and then assayed for rH] thymidine uptake as described [20]. The results are given in Table I and demonstrate little more than 10% murine MCGF activity of Pluripoietin as compared with ConA-LBRM CM (concanavalin-Astimulated conditioned media from LBRM murine lymphoma line), which was used as a standard preparation of murine MCGF. The murine Interleukin-3-dependent cell line FDC-P2 (courtesy Dr. M. Dexter) did not respond with increased [ ³H] thymidine uptake to concentrations of Pluripoietin as high as 2000 U/ml (data not shown).
Table 2 gives a summary of biological activities of Pluripoietin and Interleukin 3. Comparison is incomplete, since for some activities of Interleukin 3 on murine cells there exist no equivalent human assay systerns, as for instance long-term mast-cell line,5. From the results obtained so far, leukemia-differentiating activity is a most remarkable properly of Pluripoietin, dislinguishing it from m urine I nterleukin 3, which lacks this activity [1]. In addition, Pluripoietin is active on a wide range of hemopoietic cells, with respect to cell lineage and to their place in the hierarchy of stem cells lo malure cells. The availability of purified human hemopoielic growth factors should facilitate future studies of complex regulatory mechanisms in hematopoles IS.
I. Nicola NA, Vadas M (1984) Hemopoietic colony-stimulating factors.
Immunol Today 5:76-81 |