|
Imperial Cancer Research Fund Tumour Immunology
Unit, Department of Zoology, University College London, Gower Street,
London, WC IE 6BT, England
Interest in helper and suppressor epitopes has been growing rapidly.
It is now generally accepted that most antigens present structures
(epitopes) to the immune system which are recognized preferentially
by dif ferent sets of lymphocytes, as shown in Fig. I. These sets
belong to the effector compartment containing B cells, Tc cells
(cytotoxic cells), and delayed-type hypersensitivity-mediating T
cells (not shown), or to the regulatory compartment containing Th
and Ts cells (helper and suppressors ). Structures preferen tially
recognized by Th cells are termed helper epitopes, and so on, and
the balance of the two types of regulatory epitope is known to be
an important factor in determining the outcome of at least some
immune responses. The presence of even a single suppressor epitope
may be enough to prevent a response from occurring. The fullest
analysis of an antigen along these lines has been carried out on
lysozyme [I] and ß-galactosidase [ 18, 9], and other antigens which
have been examined in this way include ferredoxin [19] and tumour
antigens [5, 7]. Note however that cleavage of serum albumin does
not yield fragments with distinct helper and suppressor epitopes
[2, 4]. Understanding the nature of helper and suppressor epitopes
is a matter of importance to leukaemia research because it could
link tumour idiotype to membership

Fig. I. How a vaccine looks to T cells. Th- Ts repertoire
differences arc potentially valuable
of a lymphocyte set, and also because of its relevance to any future
tumour immunotherapy. It is important to immunological diseases
at large, because it is likely to help explain why particular types
of antigen tend to generate a harmful response. And at the present
time it is most obviously important to development of the new generation
of vaccines. This is an area of great excitement because these vaccines,
based on bioengineering, promise to control and eventually eradicate
the major tropical diseases. Of course one needs to exercise caution
in evaluating this promise and as yet engineered vaccines are only
just entering veterinary trials, but there is no doubt that this
hope has given new heart to vaccine research. What is now being
done, world-wide, is to take a parasite such as the plasmodium of
malaria, clone cDNA into an expression vector, and screen for antibody-defined
antigens [6, 7]. A complementary approach is to screen for antibody-reactive
synthetic peptides [ II ]. Both of these strategies concentrate
initially on the ability of vaccine molecules to interact with the
effector compartment, simply because antibodies and to a lesser
extent cytotoxic T cell clones are the only practical screening
agents. But in the long run this is too limited an approach, particularly
for the major tropical diseases all of which are long-term and characteristically
display an ineffective host response. Surely the main hope is for
a vaccine which can perturb this balance between parasite and host,
through manipulation of the regulatory compartment. The nature of
suppressor and helper epi topes is still poorly understood. I wish
here to offer a contribution towards a general theory of what makes
them different from one another. Any discussion of the problem should
start with a distinction between differences based on antigen processing
and differences based on the receptor repertoire of lymphocytes.
As regards the former, interpretations have tended to diverge, with
some authors emphasizing the importance of relatively crude factors
such as the gross anatomical localization of antigen, while others
emphasize the importance of the interaction of fragments of antigen
with particular cell receptors. Thus chemical modification can greatly
effect anatomicallocalization [3], and the route of immunization
or form in which a determinent is administered can greatly influence
which sets of lymphocyte respond [14]. On the other hand it has
been suggested that peptide fragments of antigens associate selectively
with particular major histocompatibility complex components, and
thus determine which regulatory cells respond to "aggretope selection"
[ 10]. Or suppressor cells may resemble B cells rather than helper
cells in their interaction with antigen, and consequently tend to
respond to conformational rather than sequence determinants [ 12].
These are fascinating questions, but we have little hard information
with which to answer them. In contrast there is something more definite
to say about repertoire differences between helper and suppressor
cells, even if at present this is of a rather general character.
Thanks to recent advances in our understanding of the interactions
of four
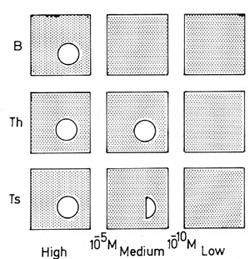
Fig.2. Only medium concentration self-macromolecules generate
Th- Ts repertoire differences (see text for details)
proteins with the immune system as they occur naturally, we can
begin to define what is probably the main factor responsible for
moulding this repertoire difference. The proteins in question are
C5, a complement component, F, a liver and serum protein of unknown
function, AFP , a-fetoprotein, and Tg, thyroglobulin. The argument
is summarized in Fig. 2, which requires some explanation. Each stippled
area represents the repertoire of antigen receptors for a set of
Iym phocytes ( each dot representing, as it were, a single clone
of cells). The circles represent holes punched out of the repertoire
by tolerance of a single self-protein; it is assumed that self-tolerance
results from purging the repertoire of clones reactive with self-proteins.
The top row describes what happens to B cells: their repertoire
is purged only by proteins which occur in the body at high concentrations,
over 10-5 M, such as serum albumin or the
constant part of immunoglobulin. The next row deals with helper
T cells: their repertoire is purged by proteins occurring down to
lower levels of concentration, 10-10
M, but no doubt there are still other molecules which occur at concentrations
too low to be noticed at all by the immune system, and for which
no purging occurs. Thyroglobulin is an example at the
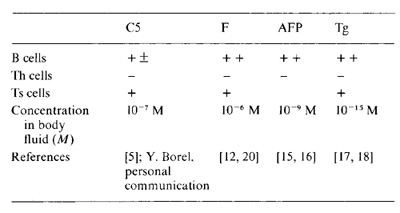
Table I. Documentation ofreactivity of the main lymphocyte
sets to four medium concentration self-proteins
borderline between the medium and low ranges of concentration: it
purges the helper cell repertoire to a significant extent, but incompletely.
At the bottom come suppressor T cells: their repertoire is no more
than partially purgcd by proteins occuring at medium concentrations,
as exemplified by the proteins F and C5. The important point is
that purging of helper and suppressor cells occurs down to different
levels, and that this difference defines a medium concentration
range at which their repertoires must differ. No other factor can
be identified which is known to generate a difference between their
repertoires. This does not mean that other factors do not operate,
but simply that at present we do not know what they are. For instance
at the time of writing there is a suspicion, but at present no more
than that, that helper and suppressor T cells draw from different
sets of V genes. The evidence for the medium concentration range
that is crucial to this argument is cited in Table 1 for the four
proteins C5, F, AFP and Tg, and is discussed in greater detail elsewhere
[13]. What are the practical consequences? It is ironic that this,
the only definite piece of information which we have about the helper-suppressor
epitope difference should produce so little in the way of practical
advice about how to design a particular epitope. Even if we knew
the full threedimensional structure of the proteins listed in Table
1 we would only be a little nearer this goal. From this point of
view research on differential antigen processing perhaps has more
to offer, even if so far its achievements have been small. For the
time being immunologists will be kept busy cloning genes and synthesizing
peptides of potential value in vaccines. More and more of these
new products will enter immunization trials without much rhyme or
reason, and as they do so we shall no doubt acquire empirical information
about which kinds of structure are good immunogens as distinct from
ones which merely react well with antibodies. It will be important
to have some kind of theoretical framework into which this information
will fit. I believe that medium concentration self-proteins as defined
here will be an important part of that framework.
References
I. Adorini L, Harvey MA, Miller A, Sercarz EE ( 1979) Fine specificty
of regulatory T cells. II. Suppressor and helper T cells are induced
by different regions of hen egg-white lysozyme in a genetically
non-responder mouse strain. J Exp Med 150:293-306
2. Benjamin DC, Daigle CA, Riley RL (1983) The antigenic structure
of bovine serum albumin. T-cell. B-cell, and la determinants. In.
Celada F. Schumacher YE, Sercarz EE (eds.) Protein confrontation
as an immunological signal. Plenum, New York, pp 261 -280
3. Dailey MO, Hunter RL (1974) The role of lipid in the induction
of hapten-specific delayed hypersensitivity and contact sensitivity.
J ImmunoII12.1526-1534
4. Ferguson TA, Peters TJr, Reed R, Pesce AJ, Michael JG ( 1983)
Immunoregulatory properties of antigenic fragments from bovine serum
albumin. Cell ImmunoI78.1-12
5. Harris DE, Cairns L, Rosen FS, Bore! Y (1982) A natural model
of immunologic tolerance. Tolerance to murine C5 is me diated by
T cells, and antigen is required to maintain unresponsiveness. l
Exp Med 156:567-584
6. Kemp Dl, Koppel RL, Cowman AF, Saim RB, Brown Gv, Anders RF (1983)
Expression of Plasmodium falciparum blood stage antigens in Escherichia
coli. detection with antibodies from immune humans. Proc Natl Acad
Sci USA 80.3787 -3791
7. Klein BY, Sharon R, Tarcic N, Naor 0 (1982) Induction orantitumour
reactive cells or suppressor cells by different molecular species
isolated from the same nonimmunogenic tumour. Immunobiology 163.7-21
8. Kong YM, Okayasu I, Giraldo AA, Beisel KW, Sundiale RS, Rose
NR, David D, Audibert F, Chedid L ( 1982) Tolerance to thyroglobulin
by activating suppressor mechanisms. Ann NY Acad Sci 392.191-209
9. Krzych U, Fowler A V, Miller A, Sercarz EE ( 1982) Repertoires
of T cells directed against a large protein antigen, ß-galactosidase.
I Helper cells have a more restricted repertoire than proliferative
cells. l Immunol 128: 1529 -1534
10. Krzych U, fowler A, Sercarz EE (1983) Antigen structures used
by regulatory T cells in the interaction among T suppressor, T helper,
and B cells. In. Celada F, Schumacher YE, Sercarz FEE (eds) Protein
confrontation an immunological signal. Plenum, New York, p 395-408
II. Lerner RA ( 1983) Synthetic vaccines. Sci Am 248.66--74
12. Lukic ML, Mitchison NA, Self and allo specific suppressor T
cells evoked by intravenous injection of F protein. Eur J ImmunoI
14.8:766-768 13. Mitchison NA, Lukic ML, Nardi N, Proteins at the
margin between being tolerated and being noticed. In. Proc Ninth
Int Covocation on Immunology. Karger, Basel 6
14. Plak W, Rozycka D, Askenase PW, Gershon RK ( 1980) Role of antigen
presenting cells in the development and persistence of contact hypersensitivity.
l Exp Med 151 :362-375
15. Ruoslahti E, Wigzell H ( 1975) Breakage of tolerance to alpha-foetoprotein
in monkeys. Nature 255.716-117
16. Ruoslahti f, Pihko H, Becker M, Makela M ( 1975) Rabbit alpha-f)etoprotein.
normal levels and breakage of tolerancc with haptenated homologous
alpha-fetoprotcin. Eur l ImmunoI5.7-10
17. Romball CG, Weigle WO (1984) Tccll competence in heterologous
and homologous thyroglobulins during thc induction of experimental
autoimmune thyroiditis fur l ImmunoI14.10:887-893
18. Turkin 0. Sercarz EE ( 1977) Key antigenic determinants in regulation
of the immune response. Proc Nat Acad Sci USA 74.3934 -3987
19. Weaver M, Singhai R, Sikora L Levy lC (1983) Identification
of an idiotypic marker of a major regulatory T cell of the immune
response in BlO.BR mice to ferredoxin. The relationship of idiotypic
regulation too conventional hapten-carrier effects. l Exp Medi 157:285-300
20. Wedderburn L, Lukic ML, Edwards S, Kahan MC, Nardi N, Mitchison
NA (1984) Single-step immunosorbent preparation of F protein from
mouse liver with conservation of the alloantigenic site, and determination
of concentration in liver and serum Mol Immunol21:10:979-984
|


