|
Midwest Children's Cancer Center. Milwaukee Children's Hospital. Milwaukee. Wisconsin. USA * This Iecture is based on work performed at St. Jude Children´s Research Hospital supported by National Cancer Institute grants CA08480. CA07594. CI\05176 CA08151. by the American Cancer Society and by ALSAC Inc work of the Midwest Children's, Cancer Center is supported by National Cancer Institute grants CA 17997. CA 17700. CA 17851. by the American Society, the Faye McBcath Foundation and the Milwaukee Athlets against Childhood Cancer Thank you for the honor of sharing in this memorial to Frederick
Stohlman. The work I will report today represents the efforts of
many physicians and scientists who have tried to understand and
control childhood leukemia. In North America and Europe acute lymphocytic
Icukcmia (ALL) represents approximately 80 percent of childhood
leukemia and 30 percent of childhood cancer. The disease usually
occurs without warning in the well child who has been well cared
for. It is characterized by fever, pallor, fatigue, malaise, bone
pain, bleeding and enlarged visccra and lymph nodes. Without effective
treatment the child soon dies of hemorrhage, injection or tumor
encroachment. The diagnosis is made by examination of aspirated
bone marrow. In 1948 it was demonstrated that antifolate compounds
produced clinical and hematological remissions in some children
with ALL [ 10]. However, the remissions were only partial cessation
of treatment was followed by relapse in a few weeks, and temporary
relapse usually occurred within a few months despite continued administration
of the drug. Subsequently , corticosteroids, mercaptopurine, vincristine
and occasionally cyclophosphamide were demonstrated to induce remissions
of a similar nature [II]. By 1961 it was possible to prolong the
lives of children with ALL for a year or more but mortality remained
near 100 percent. The major obstacles to cure were: drug resistance,
initial and acuired; inadequade distribution of drugs to the leptomeninges
resulting in primary meningeal relapse; treatment related hematosuppression,
immunosuppression and epithelial damage; and a pessimism about curing
leukemia that imprisoned the wills of many physicians [ 15, 18].
The "total therapy" plan of treating ALL initiated in 1962, embodied
several innovative features: Combination chemotherapy for induction
of remission and continuation treatment; reduction of leukemia cell
mass to subclinical levels and restoration of hematopoiesis prior
to antimetabolite therapy; meningeal irradiation early in remission
to prevent meningeal relapse; cessation of chemotherapy after 2-3
years of continuous complete remission; and most important, a purpose
to cure rather than palliate leu kemia (Table I) [15]. Early pilot
studies suggested that the plan was feasible and useful [15]. Approximately
9/10ths of the children experienced complete remission, hematological
remissions were four times the usual length, and 1/6th of the children
remained in complete remission after treatment was stopped. However,
the low doses of meningeal irradiation utilized were not effective
in preventing meningeal relapse. In a later study the meningeal
irradiation was increased and limited to the cranium and upper cervical
area, and intrathecal methotrexate was administered during the irradiation
period [2]. When followed by five-drug combination chemotherapy
for 2 1/2 to 3 years this treatment program resulted in a low frequency
of meningeal relapse and 1/2 of the children are now surviving free
of leukemia and off treatment for many years [19]. A comparative
study proved that moderately high doses of preventive craniospinal
meningeal irradiation reduced the risk of initial meningeal relapse
15 fold and again led to one-half of the children surviving free
of leukemia when they subsequently received three years of multiple
drug chemotherapy [3]. At present meningeal irradiation is the only
method demonstrated by long-term comparative study to prevent meningeal
relapse both during chemotherapy and after its cessation [3.8,14.19.21].
Most of the children who survive continuously free of leukemia for
five years and off treatment for two years are apparently cured.
In Fig. 1 the initial continuous complete remission duration of76
children entering complete remission in 1967 to 1970 are plotted
on a semilogarithmic graph. All of the children received 2400 rads
of cranial irradiation with simultaneous intrathecal methotrexate
or 2400 rads of craniospinal irradiation early during complete remission.
Subsequently they received multiple drug chemotherapy for 2lh to
3 years or until relapse or death during remission. As indicated.
the complete remission duration curve forms a plateau after 4 to
5 years. All children represented in the plateau have been in complete
remission for 8 to 10lh years and have been off treatment for 5
to 8 years. Except for the one child who relapsed after 5lh years
of complete Table I. Plan of total therapy of acute
lymphocytic leukemia 1962- 75 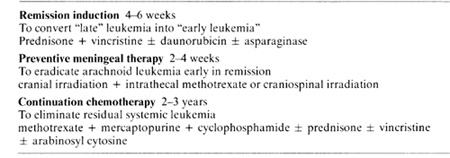 remission all children in remission at 5 years remain so. This suggests that these children are biologically different from the children in the descending portion of the curve and that this difference represents biological cure of leukemia. Since a plateau of continuous complete remission has been achieved the height of this plateau is now the criterion of success of curative treatment of ALL. Any new treatment or modification of treatment must be assessed with respect to this criterion. Since a plateau cannot be predicted or extrapolated statistically it is necessary to delay judgement about the curative value of treatment until actual experience demonstrates it. The quality of survival for most children with ALL is satisfactory (Fig. 2, 3). Within a few weeks after initiation of treatment most children can return to normal activities such as school attendance and athletics [15,19,21]. Many children have 1-2 weeks of fever and somnolence approximately 6 weeks after cranial irradiation. All the children have various degrees of hematosuppression and immunosuppression, many exhibit inhibition of skeletal growth, and some demonstrate mucosal or skin disorders, elevation of hepatic enzymes in the serum, and macrocytic anemia. The children need to be monitored carefully to avoid excessive toxicity and to control infection. Trimethoprim and sulfamethoxazole is effective in preventing Pneumocystis carinii pneumonia in children at high risk [ 12]. After termination of chemotherapy an immunological rebound may occur with lymphocytosis of the bone marrow and rise in immunoglobulin levels [6]. Hematopoiesis recovers and elevated enzymes return to normal. Often growth and weigh1 gain are accelerated and the children have increased energy and vitality [21]. Neuropsychological studies indicate that preschool children may experience impairment of short memory and 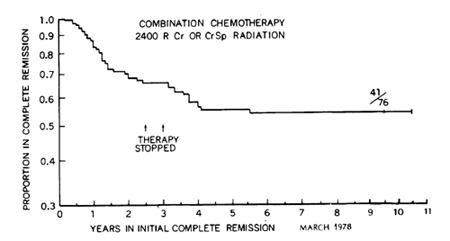 Fig.l. This semilogarithmic graph describes the initial continuous complete remi5sion duration or children who began receiving total therapy including preventive meningeal irradiation in 1967 to 1970 Treatment was stopped in all patients remaining in continuous complete remis5ion after 21h to 3 years None or the children experienced initial meningeal relapse after cessation or therapy and only one child developed relapse after five years of complete remission The level or thi5 plateau or continuous complete remission is now the measure or curative value or treatment must be established by actual experience for each treatment plan or its modification 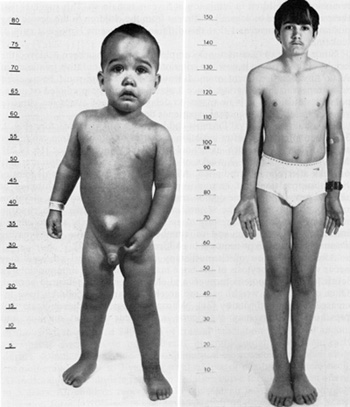
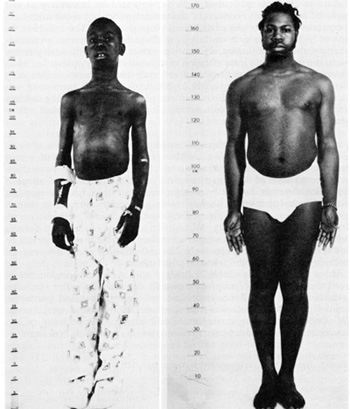
Table 2. Treatment of acute lymphocytic
leukemia. Study VIII 1972-75 
receiving methotrexate alone. On the other hand, patients receiving cyclophosphamide or cyclophosphamide and arabinosyl cytosine in addition to methotrexate and mercaptopurine tended to have shorter remissions and fewer lengthy remissions than those in the two-drug group. These results indicate that addition of simultaneous cyclophosphamide or cyclophosphamide and arabinosyl cytosine did not improve the efficacy of the methotrexate and mercaptopurine combination. Whether a cyclic or sequential schedule of two two-drug combinations might prove superior needs to be determined. The morbidity and mortality of the one, three and four-drug regimens were greater than those of the two-drug combination (Table 3). Children on methotrexate alone received two to three times higher doses of this drug than those receiving the combinations. Nine out of 20 suffered leukoencephalopathy during initial complete remission while none of the other 218 children developed evidence of this complication during initial remission. In the three- and four-drug groups immunosuppression was more pronounced and was accompanied by higher risk of varicella-Zoster infection and Pneumocystis carinii pneumonia, more frequent hospitalizations and deaths during complete remission. Thus the most efficacious treatment regimen also had the least morbidity. The most significant opportunity for improving the treatment of ALL in the past five years has been its biological and clinical classification by immunological cell surface markers (Table 4) [5,7]. This allows species identification of the leukemia cells, the first step toward developing specific cytocidal or cytostatic therapy. This may also provide further specific biological and chemical correlates of sensitivity and resistance of ALL cells to current drugs and may lead to new concepts of control of ALL. For example, the relatively good prognosis of common type ALL could be related to increased glucocorticoid receptors on the common type leukemic Iymphoblasts [22]. Other speculations for the good prognosis of common type ALL include: its origin in the bone marrow where drug diffusion is probably superior than in the visceral masses characteristic of thymic cell and B-cell ALL; its low mitotic rate and less DNA synthesis, which might reduce the risk of mutation to drug resistance; its lower number of leu - Table 3. Morbidity during initial complete
remission Study VIII 
Table 4. Immunologic clasification. childhood
lymphocytic leukemia 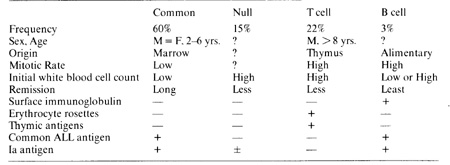
ALL in children cannot be considered incurable. Approximately one-half
of children receiving modern therapy survive free of leukemia 5-10
years after cessation of treatment and at little or no risk of relapse.
The value of any treatment program must be measured by the proportion
of children surviving free of leukemia off therapy and at little
or no risk of relapse, that is, the proportion that is apparently
cured. This cannot be projected or extrapolated from preliminary
data. The classification of ALL into biological species by immunological
markers may lead to the development of more specific and effective
treatment as well as to better understanding of its origin and nature.
Most important, it must be emphasized that the majority of children
in the world do not benefit from advances in treatment of ALL because
of their complexity, hazards, expense and inaccessibility. Therapeutic
research needs to be directed away from more complex, expensive
technology such as bone marrow transplantation and sophisticated
radiotherapy, Effort should be concentrated on understanding the
fundamental biology of children's ALL and on its practical application
for specific, effective, simple, safe and cheap treatment. In this
way we can assure that all children in the world will benefit from
our science and we can best fulfill our obligations as 5cientists
and physicians. References I. Arlin. A. A.. et al.: Therapeutic role of cell kinetics in acute
leukaemia. Clin. Haematol. 7., 339-362 ( 1978) |