|
A. Summary The discovery, characterization, and purification of human T -cell growth factor (TCGF) has led to the establishment of continuously growing T -lymphoblast cell lines from normal people and from patients with certain T -cell neoplasias. In contrast to normal T -cells, neoplastic mature T -cells respond directly to TCGF, requiring no prior lectin or antigen in vitro activation. The transformed T -cell lines have phenotypic characteristics consistent with the neoplastic cells of their disease of origin. A novel retrovirus, human T -celllymphomaleukemia virus (HTLV), has been isolated from the fresh and cultured cells of two of these patients. Subsequent characterization of this virus has shown that it is not significantly related to any known animal retrovirus, is not an endogenous (genetically transmitted) virus of man, and so far has been associated only with fresh or cultured T -cells from patients with T -cell neoplasia. These results suggest that HTLV infected some mature T -cells of some people and that it might be involved in some neoplasias involving these cells.
There are various clinical presentations of T -lymphocytic neoplasia in man, including approximately 25% of both childhood and adult acute lymphatic leukemia (Brouet et al. 197 5b ), rare cases of chronic lymphatic leukemia (Brouet et al. 1975a), and hairy cell leukemia (Saxon et al. 1978), a minority of cases of diffuse non-Hodgkin's lymphoma (a majority of cases in childhood diffuse poorly differentiated lymphoma) (Gajl-Peczalska et al. 1975; Jaffe et al. 1975), and all patients with cutaneous T -cell lymphomas (mycosis fungoides, Sezary syndrome, and nodular papulosis) (Lutzner et al. 1975). Although the Epstein-Barr virus (EBV), a DNA virus of the herpes group, is implicated in some aspect of Burkitt's lymphoma, a B-cell disease (De- The 1980), the etiology of all the T -cell neoplasias is, as of yet, obscure. RNA tumor viruses have been shown to be the etiologic agent of lymphomas and leukemias in several animal species, including chickens, mice, cats, cows, and gibbon apes ( Cockerell197 6; Gallo 1976 ; Gallo and Reitz 1976; Gallo et al. 1975; Haran-Ghera 1980; Klein 1980). In several instances these leukemias involve T -cells. When bovine leukemia virus (BLV), a causative agent of B-cellleukemia and lymphoma of cows, is injected into sheep, lymphoid leukemia and lymphoma, including a cutaneous form, occur which to our knowledge have not been subclassified (Olson 1979). It would seem reasonable then to survey human T -cell malignancies for the presence of retroviruses. Since several animal RNA tumor viruses (Klein 1980) and cells of the putative human retroviruses isolated to date (Bronson et al. 1979; Gallagher and Gallo 1975 ; Kaplan et al. 1977; Nooter et al. 1975; Panem et al. 1975) have required the establishment of continuously growing cell lines from the disease of origin or cocultivation of these cells with previously established cell lines, the ability to grow malignant T -cells in long-term culture could facilitate the isolation of retroviruses from these diseased states. We have, therefore, been interested in developing these and other cellular systems. Although there are many immunologic and cytochemical differences between mature hu man T- and B-Iymphocytes, human T -cells are primarily distinguished by their participation in cell mediated immunity and possession of receptors for sheep red blood cells. The elaboration of immunoglobulins and presence of receptors for EBV are most characteristic of human B-cells. T -cell differentiation is characterized by the successive gain or loss of certain cell surface markers and cytoplasmic enzymes which precede or coincide with the development of immunologic functions (Gupta and Good 1980). The best defined examples are: terminal deoxynucleotidyl transferase, a marker for immature or pre- T -cells; rosette formation with sheep red blood cells, a characteristic of more mature cells; and human T -cell antigens recognized by certain monoclonal antibodies on both immature and mature T -cells. In previous studies from this laboratory "activated" (by lectins) lymphoblasts from either peripheral blood or bone marrow from normal human donors were grown continuously with TCGF. Examination of these cells showed that over 90% were E-rosette positive and that all were karyotypically normal and negative for terminal transferase, EBV, and surface immunoglobulins (Morgan et al. 1976), indicating that these cells were T -lymphoblasts of relative maturity. Hence, in our initial attempts to develop cell lines from patients with T -cell neoplasias, we chose clinical subpopulations which represent a more mature form of disease, namely, the cutaneous T -cell lymphomas and leukemias and E-rosette positive T -cell ALL (Gupta and Good 1980). In this report we have summarized our recent results with TCGF, the various T -cell systems, and the isolation of anew type-C retrovirus released from growing T -cells from some of these T -cell neoplasias.
Lymphocyte reactions are complex and involve interactions between subsets of T- and B-lymphocytes and accessory adherent cells. Understanding of the regulation of the immune response has recently advanced considerably with the development of new culture methodologies (Morgan et al. 1976; Ruscetti et a]. 1977) for the long-term growth of human T-cells. Using these methods, animal and human T -cells from numerous lymphoid organs have been maintained in continuous culture for 1-3 years, provided that they were supplemented every 3-5 days with conditioned media from lectin-stimulated mononucle ar cells. Subsequent studies have shown that the agent responsible for this growth promotion is indeed a lymphokine, designated T -cell growth factor (TCGF) (Ruscetti and Gallo 1981; Smith, to be published). Thus, for the first time continuously growing clones of lymphocytes, capable of unlimited expansion in culture while retaining functional specificity and responsiveness to normal humoral regulation, were developed (Schreier et al., to be published; Smith, to be published). These cloned T -cells will be essential reagents in studies to better define the T -cell proliferative responses. The method used in our laboratory for culturing human T -cells is as follows: Leucocyte-enriched cell populations are seeded at 2-5 X 105 cells/mI suspended in tissue culture medium containing 15% heat-inactivated fetal calf serum and 20% conditioned media from PHA-stimulated leucocytes and incubated at 37°C. The cells reach their saturation density (1-2 X 106 cells/mI) in 4-5 days. It is critical that the cells are subcultured and refed with fresh Ly-CM-containing media for continued cell growth. The morphologic and functional characteristics of these cultured cells are characteristic of mature T -lymphocytes (Table 1). These cells were over 90% positive for the sheep red blood cell receptor, a test specific for T -cells, and were sensitive to human anti- T cell sera. As a test of T -cell-specific function, they responded to, but were unable to stimulate, allogeneic cells in one-way mixed leucocyte
 cultures. These cells did not contain detectable levels of terminal deoxynucleotidyl transferase, an enzyme marker for immature lymphoid cells. The population of growing cells appears to be purely T -cells, since there were no markers for other types of leucocytes. In particular, surface markers for B-lymphoblastoid cells were not detectable, including tests for surface and intracellular immunoglobulin, EBV -receptors, and B-cell-specific complement receptors. These cells could be distinguished from permanently transformed lymphoblastoid cell lines by their: (1) dependence for growth upon the continuous presence of TCGF, (2) lack of detectable EBV and surface immunoglobulins, and (3) exhibition of immunologic reactivities not associated with transformed lymphoblastoid cells. Nevertheless, in the constant presence of TCGF we have no evidence that the lifetime of these cells is finite. 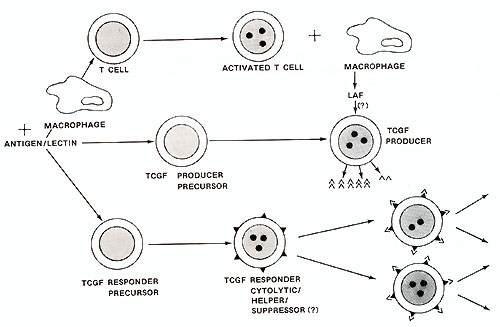
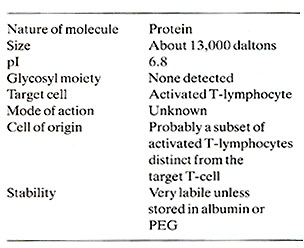
Human TCGF has been substantially purified (Mier and Gallo, 1980) and its biochemical characteristics are summarized in Table 1. The central observation concerning the control of T -cell proliferation was made using this partially purified TCGF. This was the realization that the proliferative stimulus is provided by TCGF rather than the lectin or antigen which in themselves are mitogenic only in situations where they stimulate the release of TCGF. The fact that TCGF is depleted by proliferating T -cells (Bonnard et al. 1979; Smith et al. 1980) explains the finite nature of lectin-stimulated T -cell responses and the apparent infinite proliferative capacity of T -cells continuously supplemented with TCGF . The T -cell proliferative response and acquisition of effector cell function depends upon interactions between at least three cell types as illustrated by the model in Fig. 1. The addition of antigen or lectin to a mixed population of these cell types results in a cellular activation which is characteristic for each cell. An activated adherent cell, most likely the macrophage, processes the antigen/lectin and releases a soluble product termed lymphocyte activating factor (LAF) (Oppenheim et al. 1979). This activity is not in itself a proliferative stimulus but it appears to stimulate the production and/ or release of TCGF (Larsson et al. 1980 ; Smith et al. 1980). The actual mechanism of action of LAF remains obscure. However , once the T -cells are activated and TCGF is present, the T -cells will proliferate in the absence of antigen, adherent cells, or adherent cell products. Several observations suggest that the TCGF producing-cell is a mature T -cell that is activated by antigen and stimulated by LAF to release TCGF. Highly purified T -cells produce TCGF when provided with these signals (Smith et al. 1980). TCGF production requires the maturational influence of the thymus (Gillis et al. 1979). Cloned T helper cells can produce TCGF in vitro (Smith, to be published). It is not clear whether under normal circumstances the TCGFproducer T -cell can repsond to TCGF. All the T -cell lines reported to date have required the addition of exogenous TCGF for continuous proliferation in the absence of other cells. No normal T -cell lines capable of making enough growth factor to be independent of added T -cells have been found. If the same subset of helper T -cells have the ability both to make and respond to TCGF, then it may be possible to select self -replicating helper T -cell lines. Our current views on the regulation of T -cell proliferation by TCGF is illustrated in Fig. 1. An initial result of antigen/lectin binding to the TCGF responder cell population, whether they are cytotoxic, suppressor, or helper in nature, is the acquisition of a TCGF responsive state. This responsiveness appears to be a direct result of the development of TCGF-specific membrane recpetors (Bonnard et al. 1979 ; Smith et al. 1979). Freshly isolated T -cells will neither bind nor proliferate in response to TCGF, but the addition of antigen/lectin to these T -cells will allow absorption of and proliferation in response to TCGF. The results indicate that the specificity of T -cells is restricted by the antigen that activated the cell but that the proliferate stimulus is provided by TCGF which itself has no antigenic specificity (Schreier and Tees 1980). The discovery that T -cell clonal expansion is dependent upon TCGF and is mediated through a specific receptor suggests that derangements in the immune system seen in immunodeficiency and neoplastic states can be explained by alterations in the release of or response to TCGF. In addition, agonists and antagonists of the immune system may well function by affecting TCGF production or function.
A few non-E lymphoblastoid cell lines (e.g., Molt 4, 8402, CCRF-CEM)
have been established from patients with ALL. Generally, these cell
lines are terminal deoxynucleotidyl transferase (TdT) positive,
which as noted earlier is a marker for immature lymphoblasts usually
but not necessarily restricted to the T -lymphoid lineage. These
cell lines either have no or a small percentage of E-rosette-positive
cells (Nilsson and Ponten 1975), and they neither produce TCGF nor
respond to it (our unpublished observations). We assayed some malignant
T -cells, particularly those of mature T -cell origin, for their
capacity to maintain responsiveness to TCGF. As previously discussed,
purified TCGF does not stimulate the growth of freshly isolated
normal T -cells. If the malignant T -cells were activated during
the process of transformation, these cells could then be selectively
grown by treatment with the purified TCGF. In fact, this was observed.
Long-term growth of T -cells from tissue samples from six of six
patients with cutaneous T -cell lymphoma (CTCL) and six of six patients
selected as having acute lymphocytic leukemia of a T -cell origin
was achieved by using partially purified mitogen-free TCGF (Poiesz
et al. 1980a). All these fresh samples began to proliferate after
24-48 hand were in continuous culture for at least 4 months. Some
have been maintained in culture for over a year. These cell lines
remained E-rosette positive, TdT negative, and negative for E-cell
markers, typing them as mature T-cells. The CTCL, ALL, and normal
cultured T -cells can be distinguished from one another by cytochemical
procedures. All the CTCL cell lines (and only those lines) were
strongly positive for nonspecific esterase, utilizing assay conditions
which only stain monocytes. The presence of markers for both T -cell
and monocytoid characteristics on these CTCL-cultured cells is puzzling
but probably means they are neoplastic T -cells with aberrant properties.
Normal cultured T -cells exhibited a mild granular cytoplasmic staining
for acid phosphatase which is typical of freshly isolated T -cells.
The majority of the cultured ALL cells showed a strong concentration
of the staining pattern in the Golgi region of the cells which has
been reported to be a strong indication of malignant T -cells in
fresh ALL samples (Catousky et al. 1978; Schwarce 1975). Also, in
one case, CTCL-3, the fresh and cultured cells had metaphases which
showed the same karotypic abnormalities. Also, in one case, CTCL-2,
the cells became independent of added TCGF for continuous growth
after ten passages in culture. The morphology of the cultured cells
was of interest and very similar to cells of the primary neoplasias.
For instance cultured CTCL cells contained many giant multinucleated
cells often surrounded by many mono- or binucleated smaller cells,
the nuclei of which were often convoluted ( see Fig. 2) (Poiesz
et al. 1980a). The independent growth of CTCL-2, the abnormal karyotype
of CTCL-3, the direct response to TCGF, the cytochemistry patterns
of the cell lines as consistent with studies performed on fresh
samples from patients with CTCL and ALL ( Catousky et al. 1978 ;
Schwarce 1975 ) , and perhaps most evidently, the abnormal morphology
of the cells strongly indicate that the cell lines represent the
neoplastic cell populations. We think these cell systems will be
useful for (1) comparative studies between normal and neoplastic
T -cells, (2) possible predictive value in patients in remission
by utilizing the direct response of transformed T -cells to TCGF
as an indication of the presence of residual neoplastic cells, and
( 3) providing malignant T -cells for biochemical and virological
studies relating to etiology. Their properties are summarized in
Table 2. It has been proposed that TCGF is a second signal for sustained
growth of previously activated normal T -lymphocytes (Ruscetti and
Gallo 1981). Presumably, antigen or mitogen 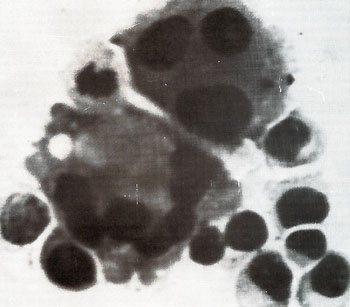
stimulation induces cell membrane alterations to produce or expose a receptor(s) for TCGF. Neoplastic T -cells may have such areceptor(s) on their cell surface at all times, thereby explaining the ability to grow T -cells from CTCL and ALL samples with pp- TCGF without prior mitogen stimulation. This could be due to either chronic stimulation by some ill-defined antigen or cellular membrane changes which lead to exposure of a receptor or its synthesis de novo. If TCGF plays a role in the in vivo regulation of T -cell replication, as seems most probably, the above model may explain a growth advantage of malignant lymphocytes over normal T -cells. Table 2. Comparative properties of continuously
cultured human T-cells 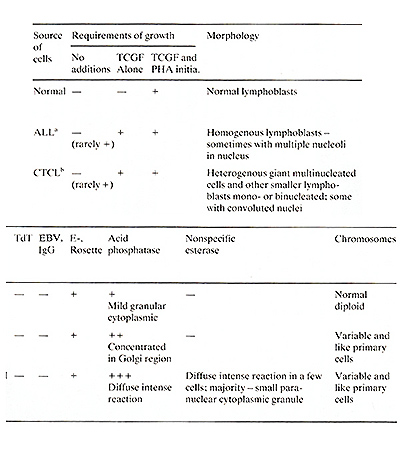
Retrovirus particles were observed budding from fresh and cultured
cells from two patients, each with a clinical variant of a cutaneous
T -cell lymphoma (Poiesz et al. 1980b, and to be published). These
viruses were subsequently isolated and characterized (see below).
Patient C.R. was a 28-year-old male with Stage IV mycosis fungoides
and patient M.B. was a 64-year-old female with the leukemic phase
of Sezary syndrome. Abnormal T -lymphoblast cell lines were derived
from both these patients using TCGF. The cell lines, HUT102 ( Gazdar
et al. 1980; Poiesz et al. 1980a) and CTCL-3 were established 1
year apart from the right inguinal lymph node and peripheral blood
of patient C.R. Another cell line, CTCL-2, was derived from aleukemic
peripheral blood sample from patient M.B. (Poiesz et al. 1980).
HUT102 and CTCL-2 cells are now grown independent of added TCGF,
but CTCL-3 still requires it. Morphologically typical (Schidlovsky
1977) type-C budding, immature, and mature virus particles have
been observed on electron micrographs of cell pellets from all three
of these cell lines and fresh peripheral blood lymphocytes from
patient C.R. (Fig. 3). Initially, virus production from HUT102 cells
required prior induction iododeoxyuridine (IDUR) but spontaneously
became a constitutive producer of virus at a later passage, whereas
CTCL-3 cells have always been con 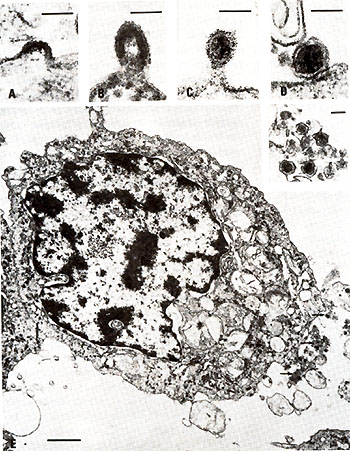
stitutive producers. CTCL-2 cells have always required IDUR induction of virus. Typical of a retrovirus, the HTLV isolates band at a density of 1.16 gm/ml in continuous sucrose gradients (Poiesz et al. 1980, and to be published), contain 70S RNA (Reitz et al., to be published) and are associated with a DNA polymerase which prefers the template primers poly rA.oligo dT and poly rC.oligo dG over poly dA.oligo dT. Purified DNA polymerase from HTLV CR has shown the same results with the above template primer and has been demonstrated to catalyze transcription of purified simian sarcoma virus (SSV) 70S RNA and human mRNA (Rho et al., to be published). These are all characteristics of a viral reverse transcriptase (RT) (Sarngadharan et al. 1978). The molecular weight of purified HTLV RT is about 95,000. The enzyme shows preference for Mg+ + as its divalent cation, especially with the template-primer, poly rC.oligo dG (Kalyanaraman et al., to be published). This combination of morphologic and biochemical characteristics are atypical for most known animal viruses; HTLV does not easily fit into a clear type C, B, or D pattern. Rather, it is suggestive of those viruses which are difficult to classify, e.g., BLV (Olson 1979) or the particle-associated RT -like activity found in some fresh human placentas (Nelson et al. 1978). The major protein bands of disrupted purified HTLV CR and HTLV MB particles as determined by SDS polyacrylamide gel electrophoresis are identical and have mol. wts. of approximately 81,000 (p81), 52,000 (p52), 42,000 (p42), 24,000 (p24), 18,000 (p18), 12,000 (p12), and 10,000 (p10) (Reitz et al., to be published; Rho et al., to be published). These proteins are consistent in size and number with that expected of a retrovirus (August et al. 1974). These same proteins bands are identifired when HUT102 cells are grown in H³-leucine and the subsequently isolated and purified HTLV CR particles are disrupted and examined by SDS PAGE. Hence, they represent either viral or cellular proteins rather than a contaminant from the fetal calf serum in which the cells are grown. Several proteins, p81, p24 and p 18 are labeled with Il25 only after disruption of HTLV CR particles with detergent and, therefore, probably are viral core proteins (Kalyanaraman et al., to be published). We think p24 is the major structural core p30 of HTLV because of its relative quantity, molecular weight, elution profile on phosphocellulose (Kalyanaraman et al., to be published and see below), and co-purification with viral cores.
As is evident from the above discussion the HTLV isolates can be categorized as retroviruses because they have retrovirus morphology and mode of budding from cell membranes, a density of 1.16 g/ml by sucrose gradient analysis, and contain 70S RNA, structural proteins analogous to retrovirus proteins, and a DNA polymerase. There are four independent cell sources from two different patients which release HTLV; all were grown in culture in the presence of TCGF. Two of these cell lines (HUT-102 and CTCL-2) have become TCGF independent, apparently because they produce their own TCGF. These two cell lines are producers of HTLV. The characterization of the HTLV DNA polymerase clearly indicates that it is a RT. Prior to its purification HTLV RT catalyzes an endogenous DNA synthesis. The cDNA product can be isolated and purified. It completely (>90% ) hybridizes back to purified HTLV 70S RNA (see Reitz et al. in this book). Purified HTLV RT catalyzes transcription of purified viral70S RNA (or mRNAs) in reconstituted reactions (Reitz et al., to be published). Purified HTL RT utilizes poly rC.oligo dG and poly rA.oligo dT, but not poly dA.oligo dT (Rho et al., to be published) -characteristics of a retrovirus RT (Gallo and Reitz 1976) ; Gallo et al. 1975; Sarngadharan et al. 1978). Purified HTLV RT is about 95,000 daltons, shows preference for Mg+ + for its divalent cation, and of all synthetic template primers, utilizes poly rC.oligo dG most efficiently (Rho et al., to be published). As noted above these characteristics mimic the diffecultto-classify retroviruses, i.e., those not clearly type C, D, or B, e.g., (BLV). Several analyses of HTLV have been completed. All of these results show that HTLV is not closely related to previously isolated animal retroviruses. These results are summarized here.
As noted above, HTLV RT has been purified. We (Rho et al., to be published) have compared purified HTLV to other RTs purified from animal retroviruses for immunologic relatedness. We have described these types of assays previously at these meetings in other comparative studies (Gallo 1976; Gallo 1979). Briefly, we have made antibodies to RTs from many animal retroviruses by inoculating goats or rats with the purified RT (Mondal et al. 1975 ; Smith et al. 1975; Todaro and Gallo 1973). The hyperimmune sera are obtained, and in most cases they strongly neutralize the DNA polymerase activity of the homologous RT. These antisera also generally show cross reactions which are in keeping with the known relatedness of lack of relatedness of different retroviruses as determined by other types of comparisons. Sometimes neutralization of polymerase activity of the homologous enzyme is not obtained. In these cases, however, a positive and specific binding of the antibody to the RT can be demonstrated (Robert-Guroff and Gallo 1977; 1979). When these tests were made with RT from HTLV no detectable cross reactons was found with any of the antisera to animal retroviruses (Poiesz et al. 1980b; Rho et al., to be published). These results are summarized in Table 3. Table 3. Lack of detectable relatedness
of purified reverse transcriptase of HTLV 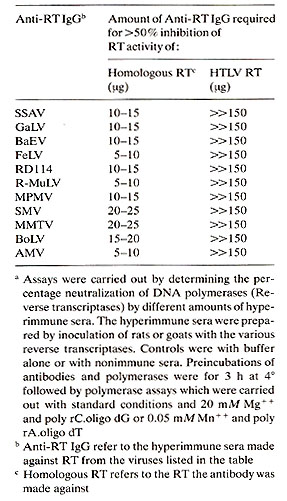
The major internal protein of HTLV has a molecular weight of 24,000 (Kalyanaraman et al., to be published). This protein is analogous to the major core protein (p24 to p30) of animal retroviruses. The evidence that HTLV p24 is a viral protein of HTLV and not a cellular or serum protein contaminating the virion preparations is as follows : 1. The p24 is the major protein associated with HTLV. , 2. p24 copurifies with HTLV cores and increa ses as virus titer is increased ; 3. p24 has the same biochemical behavior as animal retrovirus core proteins (p24 to p30), e.g., size and characteristics of elution from phosphocellulose columns; and 4. p24 is readily detectable in the neoplastic human T -cells producing HTLV but not in normal human cells, including normal growing human T -cells. These observations are all reported in detail elsewhere (Kalyanaraman et al., to be published). The p24 of HTLV is not significantly related to proteins of animal retroviruses. The evidence for this is summarized here. Hyperimmune serum was obtained against p24 of HTLV (Kalyanaraman et al., to be published). This antibody precipitates 1125-labeled HTLV p24, but not significantly proteins of animal retroviruses. Conversely, antibodies to p24-p30 of various animal retroviruses do not significantly precipitate HTLV 1125-p24. Competition radioimmune assays were next employed. None of the tested animal retroviruses p24-p30 competed in precipitation of HTLV 1125-p24 by HTLV p24 antisera, while cold HTLV competed completely. Conversely, HTLV p24 did not compete in various homologous radioimmune precipitation assays using 1125 p24-p30 of animal retroviruses and their corresponding antisera. For example, whereas 10 to 100 µg of unlabeled p30 from SSA V, BaEV, or MuLVRauscher competed 50% of the precipitation of their 1125-p30 by the corresponding antisera, unlabeled HTLV p24 did not compete. Finally, p30 of certain retroviruses are known to contain interspecies determinants. These cross reactions can be detected by heterologous competitive radioimmune assays, i.e., by using 1125 p30 of one virus and antisera to p30 of another (related) virus. These assays show, for example, that p30 of some mammalian retroviruses are closely related (reviewed in Aaronson and Stephenson 1976). We have confirmed the reported relatedness of some of the Type-C mammalian retroviruses, and we have shown that HTLV p24 does not compete in these assays. These results are reported in detail elsewhere (Kalyanaraman et al., to be published), and a list of the animal retroviruses tested for lack of p24-p30 relatedness to HTLV p24 is presented in Table 4.
Table 4. HTLV p24 is distinct from p24-p30
of the animal retroviruses listed herea 
The relatedness of HTLV nucleotide sequences to those of animal
retroviruses was examined by several approaches. These results show
a very slight but reproducible homology between HTLV and sequences
from viruses of the
In vitro and in vivo experiments are in progress to determine whether HTLV can infect certain cell types or effect their growth. So far HTLV have not been transmitted to any of several cell types from different animals including humans. The results to date suggest that either the HTLV isolates are in some way defective or that cell receptors for them are unusual and yet to be found. The two suggestions are not mutually exclusive.
Many animal retroviruses are endogenous to a given species, that is, their genomes are present in the DNA of all tissues of most and in some cases possibly all members of a species. They are not generally infectious to the species but are transmitted in the germ line in a Mendelian genetic manner. These retroviruses are often non oncogenic in contrast to BLV, GaLV, FeLV, AMV, etc. which cause leukemias and lymphomas by some kind of infection. We do not know how HTLV is transmitted. It is possible that it is endogenous and vertically transmitted in the germ line of select familiesl. However, we can conclude that it is not a widespread endogenous genetically transmitted virus of humans because ³H-cDNA and I125- 70S RNA of HTLV does not hybridize to DNA purified from normal human tissues. Over 30 samples were examined and none contained detectable HTLV sequences under conditions that would readily detect one copy per haploid genome. These results are also summarized by Reitz et al. elsewhere in this book and will be reported in detail in a separate publication (Reitz et al., to be published). 1 Note added in proof: New results have shown that sequences of HTLV can not be found in cultured normal B-cells from patient C. R. Therefore, this virus in the neoplastic T -cells must be acquired not genetically transmitted
There is now substantial evidence that HTLV was present in the primary tissues or leukemic blood cells of some of the patients we have had the opportunity to study. The evidence summarized here is as follows. 1. HTLV nucleotide sequences were found in the DNA of the fresh leukemic cells of patient M.B., the patient with Sezary leukemia from which cell line CTCL-2 was established (Poiesz et al., to be published). As noted earlier, CTCL-2 releases virus ( called HTLV MB) very similar to the first isolate of HTLV (Poiesz et al., to be published). 2. HTLV nucleotide sequences were found in the DNA of uncultured leukemic cells of a 16 yr. old young man with T-cell ALL. Some of these results are summarized by Reitz et al. in this book and published in detail elsewhere (Reitz et al. 1981). 3. Extracts of the fresh leukemic cells of patient M.B .competed for the radioimmune precipitation of 1125 HTLV -p24 by its homologous antisera, suggesting that HTLV p24 was in the fresh leukemic cells of patient M.B. (Poiesz et al., to be published) and 4. Antigens detected by HTLV antibodies and antibodies reactive with HTLV proteins have been found in some other patients by M. Robert-Guroff and L. Posner in our laboratory. We have not yet found HTLV, antigens, or HTLV nucleic acids in normal cells or in cells or tissues derived from patients with myeloid leukemias, B-cell leukemias, or carcinomas. Our evidence to date then associates HTLV only with neoplastic and relatively mature T -cells of some patients. Therefore, our working hypothesis is that HTLV is an unusual infection of humans with a very specific target cell.
HTLV are novel retroviruses which are found in some human mature T -celllymphomas and leukemias. We think they are an unusual infection with very specific target cells. They may act on those subsets of T -cells which are able to produce TCGF. This interaction might allow for abnormal TCGF release which in turn leads to abnormal proliferation, a model similar to the proposed model made previously at these meetings (Gallo 1979). A wide epidemiological survey by more than one sensitive technique is now needed to further understand the possible role of this virus in human disease.
Aaronson SA, Stephenson JR (1976) Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta 458: 323-354 - August JJ, Bolognesi DF, Fleessner I, Gilden RV, Nowinski RC (1974) A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology 60: 595-605 -Bonnard GD, Yasaka K, Jacobson D (1979) Lectin-activated T -cell growth factor-induced proliferation: Absorption of T -cell growth factor by activated T-cells. J Immunol 123 :2704-2709 -Bronson SL, Fraley EE, Fogh J, Kalter SS (1979) Induction of retroviral particles in human testicular tumor (Tera-1) cell cultures. An electron microscopic study. J Natl Cancer Inst 63: 337-339 -Brouet JC, Flandrin G, Sasportes M, Freud-Homme JL, Seligmann M (1975a) Chronic lymphocytic leukemia of T -cell origin, immunological evaluation in eleven patients. Lancet 2: 890-893 -Brouet JC, Freud 'Homme JL, Seligmann M ( 197 5b ) The use of band T membrane markers in the classification of human leukemias with special reference with acute lymphocytic leukemia. Blood Cells 1: 81-90 -Catousky D, Cheschi M, Greaves MF (1978) Acidphosphatase reaction in acute lymphoblastic leukemia. Lancet 1:749-751 -Cackerell GL (1976) Characterization of feline T and B lymphocytes and identification of experimentally induced T cell neoplasias in the cat. J Natl Cancer Inst 57:907-914 -De-The' G (1980) Epstein-Barr virus in human diseases: Infectious mononucleosis, Burkitt's lymphoma and nasopharyngeal carcinoma. In: Klein G (ed) Viral oncology. Raven, New York, pp 769- 798 -Gajl-Feczalska K, Bloomfield CD, Sasein H (1975) Analysis of blood and lymph nodes in 87 patients. Am J Med 59: 674-685 -Gallagher RE, Galla RC (1975) Type-C RNA tumor virus isolated from cultured human acute myelogenous leukemia cells. Science 187 :350-353 -Gallo RC (1976) RNA tumor viruses and leukemia: Evaluation of present results supporting their presence in human leukemias. In: Neth R, Galla RC, Mannweiler K, Maloney WC ( eds ) Modern trends in human leukemia II. Lehmanns, Germany, pp 431-450 - Gallo RC ( 1979) Cellular and virological studies directed to the pathogenesis of the human myelogenous leukemias. (The First Frederick -Stahlman memorial lecture) In: Neth R, Galla RC, Hofschneider FH, Mannweiler K ( eds ) Modern trends in human leukemia III. Springer, Berlin Heidelberg New York, pp 7-24 -Gallo RC, Reitz MS (1976) Molecular probes for tumor viruses in human cancer . Int Rev Exp Pathol 16: 1-58 -Gallo RC, Gallagher RE, Miller NR, Mondal H, Saxinger WC, Mayer RJ , Smith RG, Gillespie DH (1975) Relationships between components in primate RNA tumor viruses and in the cytoplasm of human leukemic cells : Implications to leukemogenesis. Cold Spring Harbor Symp Quant BioI 39 : 933-961 - Gazdar AF, Carney DN, Bunn PA, Russell EF, Jaffe ES, Schechter GP, Guccion JG ( 1980) Mitogen requirements for the in vitro propagation of cutaneous T cell lymphomas. Blood 55 :409-417 - Gillis S, Baker PE, UnionNA, Smith KA (1979) The in vitro generation and sustained culture of nude mouse cytolytic T -lymphocytes. J Exp Med 149: 1460-1466 - Gupta S, Good RA (1980) Markers of human lymphocyte subpopulations in primary immunodeficiency and lymphoproliferate disorders. Semin Hematol 17: 1-29 -Guroff MR, Gallo RC (1977) Serological analysis of cellular and viral DNA polymerases by an antiserum to DNA polymerase µ of human lymphoblasts. Biochemistry 16:2874-2880 - Guroff MR, Gallo RC (1979) Type-specific binding antibody to baboon endogenous virus (M7) reverse transcriptase. J Gen Virol 43: 1-6 -Haran-Ghera N ( 1980) Pathogenesis of murine leukemia. In: Klein G (ed) Viral oncology. Raven, New York, pp 161-185 -Jaffe ES, Shevach EM, Sussman EH (1975) Membrane receptor sites for the identification of lymphoreticular cells in benign and malignant conditions. BrJ Cancer 31: 107-120 (Suppl) -Kalyanaraman VS, Sarngadharan MG, Poiesz B, Ruscetti FW, Gallo RC (to be published) Immunological properties of a type-C retrovirus isolated from cultured human T -lymphoma cells and comparison to other mammalian retroviruses. -Kaplan HS, Goodenow RS, Epstein AL, Gartner S, Decleve A, Rosenthal PN (1977) Isolation of type-C RNA virus from an established human histiocytic lymphoma cell line. Proc Natl Acad Sci USA 74:2564-2568 -Klein G (1980) Viral Oncology. Raven, New York-Larsson EL, Iscove NN, Coutinko A (1980) Two distinct factors are required for induction of T -cell growth. Nature 283: 664-666 -Lutzner M, Edelson R, Schein P, Green I, Kirkpatrick C, Ahmd A (1975) Cutaneous T -cell lymphomas: The Sezary syndrome, mycosis fungoides and related disorders. Ann Intern Med 83 :534-552 -Mier JW, Gallo RC (1980) Purification and some characteristics of human T -cell growth factor (TCGF) from PHA-stimulated lymphocyte conditioned media. Proc Natl Acad Sci USA 77 :6134-6138 -Mondal H, Gallagher RE, Galla RC (1975) RNA-directed DNA polymerase from human leukemic blood cells and from primate type-C virus producing cells: High and low molecular weight forms with variant biochemical and immunological properties. Proc Natl Acad Sci USA 72:1194-1198 - Morgan DA, Ruscetti FW, Gallo RC (1976) Selective in vitro growth of T -lymphocytes from normal human bone marrows. Science 193: 1007-1008 - Nelson J, Yeong J, Levy J (1978) Normal human placentas contain RNA-directed DNA polymerase activity like that in viruses. Proc N atl Acad Sci USA 75 : 6263-6267 -Nilsson K, Ponten J ( 1975 ) Classification and biologic nature of established human hematopoietic cell lines. Int J Cancer 15:321-341 -Nooter K, Aarssen AM, Bentvelzen P, d'Groot FG (1975) Isolation of an infectious C-type oncomavirus from human leukemic bone marrow cells. Nature 256: 595-597 - Olson C ( 1979) Progress for control of bovine leukosis. Practitioner 14:115-120 - Oppenheim JJ, Mizel SB, Meltzer MS (1979) Biological effects of lymphocyte and macrophage-derived mitogenic "amplication" factors. In: Cohen S, Peck E, Oppenheim JJ (eds) Biology of the Lymphokines. Academic, New York, pp 291-323 - Panem S, Prochownik EV, Reale FR, Kirsten WH (1975) Isolation of C-type virions from a normal human fibroblast strain. Science 189:297-299 -Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC (to be published a) Isolation of type-C retrovirus particles from cultured and fresh lymphocytes of a patient with cutaneous T -cell lymphoma. Proc Natl Acad Sci USA -Poiesz BJ, Ruscetti FW, Mier JW, Woods AM, Galla RC (1980) T cell lines established from human T -lymphocytic neoplasias by direct response to T -cell growth factor. Proc Natl Acad Sci USA 77:6815-6819 -Poiesz BJ, Ruscetti FW, Reitz MS, Kalyanaraman VS, Galla RC ( 1980) Evidence for nucleic acids and antigens of a new type-C retrovirus (HTLV) in primary ulcultured cells of a patient with Sezary T -cell leukemia and isolation of the virus. Proc Natl Acad Sci USA 77:7415-7419 - Reitz MS JR, Poiesz BJ, Ruscetti FW, Galla RC ( 1981) Characterization and distribution of nucleic acid sequences of a novel type-C retrovirus isolated from neoplastic human T -lymphocytes. Proc Natl Acad Sci USA 78:1887-1891 -Rho HM, Poiesz B, Ruscetti FW, Galla RC (to be published) Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T -cell lymphoma cell line. -Ruscetti FW, Galla RC (1981) HumanT-lymphocyte growth factor: Regulation of growth and function of T -lymphocytes. Blood (Ed. Review) 57:379-394 - Ruscetti FW, Morgan DA, Galla RC ( 1977) Functional and morphological characterization of human T cells continuously grown in vitra. J Immunal 119:131-138 -Sarngadharan MG, Rabert-Guraff M, Galla RC (1978) DNA polymerases of normal and neoplastic mammalian cells. Biochim Biophys Acta 516:419-487 -Saxon A, Stevens RH, Galde DW (1978) T-lymphocytic variant of hairy cell leukemia. Ann Intern Med 88:323-326 -Schidlovsky G (1977) Structure of RNA tumor viruses. In: Galla RC ( ed) Recent advances in cancer research: Cell biology, molecular biology and tumor virology, Val I. CRC, Cleveland Ohio, pp 189-245 -Schreier MH, Tees R (1980) Clonal induction of helper T cells: Conversion of specific signals into nonspecific signals. Int Arch Allergy Appl Immunol61 :227-231 - SchreierMH, Iscove NN, Tees R, Aardon L, von Boehmer H ( to be published) Clones of killer and helper T cells : Growth requirements, specificity and retention of functions in long-term culture. Immunol Rev -Schwarce WW (1975) T cell origin of acid-phosphatase positive lymphoblasts. Lancet 2: 1264-1266 -Smith KA (to be published) T-cell growth factor. Immunol Rev -Smith RG, Abrell 1W, Lewis B1, Gallo RC (1975) Serological analysis of human deoxyribonucleic acid polymerases. 1 BioI Chem 250: 1702-1709 - Smith KA, Lachman LB, Oppenheim 11, Favata MF (1980) The functional relationship of the interleukemias. 1 Exp Med 151: 1551-1555 -Todaro G1, Gallo RC (1973) Immunological relationship of DNA polymerase from human acute leukaemia cells and primate and mouse leukaemia virus reverse transcriptase. Nature 244:206-209 |