Hämatol. Bluttransf. Vol 29 |
|
German Multicenter Trial for Adult ALL/ AUL, FRG. Supported by the Bundesministerium für Forschung und Technologie. Contract No.01 ZW 450
The German Multicenter Trial for Adult Acute Lymphoblastic (ALL) and Acute Undifferentiated (AUL) Leukemia was undertaken to improve remission duration by using a modified form of an intensified induction regimen successful in childhood ALL (Riehm et al. 1980). The results from the pilot study, with a total of 162 patients and a median observation time of 41/2 years, now allow some conclusions regarding prognostic factors which influence the achievement of complete remission or the length of remission.
The concept of this therapeutic trial was to eradicate as much as possible of the initial tumor cell load by an eight-drug induction therapy and a similarly intensive early consolidation therapy after 3 months, whereas the maintenance therapy is conventional with 6-mercaptopurine and methotrexate. The 8-week induction regimen consists of two phases: In the first 4 weeks prednisone 60 mg/m² PO daily, vincristine 1.5 mg/m² IV once weekly, daunorubicin 25 mg/m² IV once weekly, and l-asparaginase 5000 units/m² IV on days 1-14; and in the second 4 weeks cyclophosphamide 650 mg/m² IV 3 doses at 2-week intervals, cytosine arabinoside 75 mg/m² IV on 4 days per week for 4 weeks, and 6-mercaptopurine 60 mg/m² PO daily for 4 weeks. CNS prophylaxis consists in methotrexate 10 mg/m² intrathecally each week and CNS irradiation with 24 Gy. A 6-week reinduction course is given after 3 months and is similar to the induction regimen, adriamycin being substituted for daunorubicin, dexamethasone for prednisone, and thioguanine for 6-mercaptopurine; L-asparaginase is omitted. Maintenance therapy with 6-mercaptopurine 60 mg/m² PO daily and methotrexate 20 mg/m² PO or IV once weekly is continued for 2 years. Further details of the therapy and of the diagnostic procedure have been described previously (Hoelzer et al. 1984).
From October 1978 to June 1981 a total of 162 adult patients from 25 hospitals entered the study, and 126 (77.8%) achieved complete remission. At the evaluation date, 30 November 1983, the median survival time for all patients was 23.4 months and that for complete remitters was 34 months. Median remission duration was 20.5 months. The probability of being in complete remission at 4 1/2 years is 0.397. Cell marker analysis identified c-ALL in 56.4%, null-AL (defined as being non-B-ALL, non-T-ALL, cALLA-) in 25.6%, T-ALL in 15.4%, B-ALL in 0%, and mixed leukemia in 2.6%.The best results were achieved in patients with T -ALL for whom the probability of being in continuous complete remission at 4 1/2 years is 0.636. Table 1. Prognostic factors for remission
duration: Pilot study 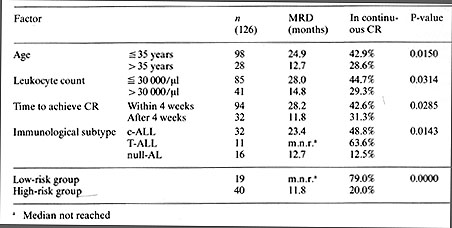
Regarding the complete remission rate. none of the initial laboratory
or clinical features, such as age, leukocyte count, hepatosplenomegaly,
mediastinal tumor, CNS involvement, or other organ infiltration,
had an unfavorable influence on the achievement of complete remission.
The adverse effect of older age, high initial leukocyte count, and
hepatosplenomegaly, which have been shown in other studies to have
an unfavorable influence on the achievement of complete remission,
could not be confirmed in the large number of patients in this study
(Hoelzer et al. 1984).
The study group has developed anew riskadapted therapy protocol based on the results of the pilot study, which was activated on I July 1983. According to this, in addition to the intensive induction therapy and consolidation therapy, high-risk patients will receive further cycles of consolidation therapy with VM-26 and cytosine arabinoside, to improve results in this group. In addition, the high-risk patients are to be considered for allogeneic bone marrow transplantation in first remission if a suitable donor is available. After establishment of the method, autologous bone marrow transplantation also appears to be useful for these patients. The low-risk patients will be treated according to the present protocol with no essential changes. It is to be expected that in this group, even with chemotherapy alone, more than 50% will reach the 5-year limit without disease and might thereby be considered as cured.
Frei E, Sallan SE (1978) Acute lymphoblastic leukemia. treatment.
Cancer 42:828-838 |