|
When a mixture of erythrocytes (RBCs) and a foreign substance is dialysed against a hypotonic saline, the hemoglobin is replaced with the substance (Furusawa et al. 1976 ). The resultant ghosts containing foreign substances could be used for various therapeutic purposes.
Fusion between cells and RBC ghosts containing foreign substances results in the introduction of the substances into the cells (Furusawa et al. 1974 ). With this technique any substance smaller than 6 X 10 high 7 dalton can be injected into the cytoplasm. An accurate quantitative injection can be performed using a combination of this method together with a cell sorter (Yamaizumi et al. 1978). It is significant that there is no limitation in the number of target cells subjected to microinjection. This fusion injection technique could be used for alleviating or repairing defective cells. That is, drugs, nucleic acids, or enzymes of normal counterparts can be introduced into these cells, then injected back to the patient. Because the cells and RBCs used can be obtained from the individual to be injected, there would be no risk of rejection (Furusawa 1980). In spite of such advantages it has an inevitable weak point in that intranuclear injections are impossible. Recently we developed a new instrument, namely an "injectoscope", by which one can easily perform intranuclear microinjections without employing a conventional micromanipulator (Furusawa et al. 1980; Yamamoto and Furusawa 1978).
RBC ghosts loaded with drugs may serve as a good tool for the treatment of some diseases, especially hepatic diseases. When mouse RBC ghosts loaded with proteins were injected into a vein, they disappeared from the circulation blood within 30 min and accumulated exclusively in the liver and spleen. The transfused ghosts may be trapped by Kupffer's cells or macrophages where the loaded substance might be liberated. In clinical therapy a specific drug for a given disease can be loaded in the RBCs of the patient. Then, the drug-Ioaded ghost cells could be injected intravenously to the same patient. Thus a concentrated amount of the drug could be administered to the liver. Accordingly, it can be expected to diminish subsidiary ill effects of the drug. The fate of the drug thus introduced remains to be examined.
In humans two important requirements for a successful immunization are ( I) a high antibody producing efficiency and (2) minimized side effects. Our preliminary study demonstrated that the intraperitoneal injection of RBC ghosts loaded with dinitrophenol-conjugated ovalbumin (DNP-OA) gave rise to a significant increase of anti-DNP antibody production without artificial adjuvant. The introduction of DNP-OA into the ghosts was carried out as follows: A mixture of I volume of packed RBCs from ICR mice and 9 volumes of PBS containing 10 mg/ml of DNP-OA was dialysed to ten fold diluted PBS until hemolysis had been accomplished and followed by dialysis against isotonic PES. In addition, a sample containing 1 mg/ml of DNP-OA entrapped in 2 x10 high 9 ghosts was prepared. For sensitization the ghosts loaded with DNP-OA were intraperitoneally injected into additional mice of the same strain. For controls, free DNP-OA, a mixture of vacant ghosts and DNP-OA, vacant ghosts, and an emulsion with Freund's complete adjuvant were injected. The antisera were collected on the 11th day. The second sensitization was performed on the 11 th day after the first injection, and the sera obtained after an additional 11 days. A passive hemagglutination (PHA) test was used for the titration of serum antibody (anti-DNP). The result is summarized in Table 1. Compared with free DNP-OA, ghosts loaded with DNP-OA resulted in a significant increase of anti-DNP production, even in the primary response. In the secondary response higher titers were obtained when the ghosts loaded with DNP-OA were used, although the titers were lower than those with the emulsion. The most striking difference is seen between the sensitization with free DNP-OA and the ghosts loaded with DNP-OA of 25µg in the secondary response. no detectable antibody was obtained in the former while 2 high 4 to 2 high 9 in the titer were observed in the latter. The present experiment clearly shows that sensitization with antigens entrapped in the REC ghosts produces a significant amount of antibodies without adjuvant when intraperitoneally introduced. An in vitro study suggests that antigen-Ioaded ghosts are positively engulfed by abdominal macrophages. If so, the leakage of antigens from the ghosts is minimized. This would raise the antibody forming efficiency and diminish subsidiary ill effects involved. As neither adjuvants nor artificial carriers such as liposomes (Poste et al. 1976 ) are used in the present method, their possible harmful effects can be avoided. Table 1. Anti-DNP antibody production
in mice receiving intraperitoneal injections of RBC ghosts loaded
with DNP-OA as compared to the positive and negative controls (lino
& Furusawa, unpublished)a 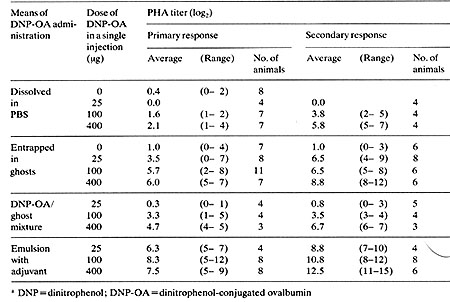
Furusawa M ( 1980) Cellular microinjection by cell fusion Technique and applications in biology and medicine. Int Rev Cytol 62:29-67- Furusawa M, Nishimura T. Yamaizumi M, Okada Y (1974) Injection of foreign substances into single cells by cell fusion Nature 249:449-450 -Furusawa M, Yamaizumi M, Nishimura T, Uchida T, Okada Y (1976) Use of erythrocyte ghosts for injection of substances into animal cells by cell fusion Methods Cell Biol 14:73-80- Furusawa M, Yamamoto F, Hashiguchi M, Swetly P, Zlatanova J ( 1980) Studies on Friend cell differentiation using a new microinjection technique In' Rossi GB (ed) In vivo and in vitro erythropoiesis' Thc Friend system Amstcrdam, Eisevier/North-Holland Biomedical Press, pp 193-198 -Poste G, Papahadjopoulos D, Vail W ( 1976) Lipid vesicles as carrier for introducing biologically active materials into cells Methods Cell Biol 14:33-71 -Yamaizumi M, Mekada E, Uchida T, Okada Y (1978) One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 15: 245-250 -Yamamoto F, Furusawa M (1978) A simple microinjection technique not employing a micromanipulator Exp Cell Res 117:441-445 |