|
1 Imperial Cancer Research Fund Department of Medical
Oncology. St. Bartholomew's Hospital. and Lahoratory of Membrane
immunology. Lincoln's inn Fields. London
2 Department of Haematology. St. Bartholomew's Hospital. London
Introduction
The introduction of three and four drug combination chemotherapy
into the treatment of acute lymphoblastic leukemia (ALL) in adults
has resulted in complete remission being achieved in approximately
70% of cases (Willemze. Hartgrink-Groeneveld, 1975; Jacquillat and
Weil, 1973; Gee and Haghbin. 1976; Muriel and Pavlovsky, 1974; Atkinson
and Wells. 1974; Einhorn and Meyer, 1975; Rodriguez and Hart, 1973;
Whitecar and Bodey. 1972; Spiers and Roberts. 1975). In spite of
the use of early central nervous system prophylaxis and continuous
maintenance chemotherapy. however. the duration of complete remission
remains considerably shorter than in childhood ALL. It is well documented
that certain presentation features influence the prognosis in childhood
(Henderson, 1969; Simone and Holland. 1972). We have. therefore.
analysed the data from 42 adults in whom complete remission was
achieved to determine which presentation features influence the
prognosis in adults.
Materials and Methods
A. Patients
Between November 1972 and December 1976. /62 consecutive previously
untreated adults with ALL were treated with combination chemotherapy
at St. Bartholomew's Hospital. All patients received adriamycin,
vincristine. prednisolone and L-asparaginase, as previously reported
(Lister. Whitehouse. 1978). and complete remission was achieved
in 43 (69%). One patient returned to India without maintenance therapy
and subsequently relapsed. The remaining 42 cases form the basis
of this analysis. All recieved early central nervous system therapy
and continuous maintenance chemotherapy until relapse or for three
years. whichever was shorter.
B. Diagnostic Criteria
The diagnosis of ALL was based upon conventional morphological criteria
(Bennett and Catovsky. 1976) for May-Grunwald-Giemsa stained bone
marrow smears. which showed at least 30% infiltration by agranular,
Sudan Black negative blast cells. The periodic-acid-Schiff (PAS)
stain was performed in all cases and considered positive if more
than 5% of the blasts exhibited block or coarse granular activity.
Cases with an occasional fine granular or negative reaction were
also negative for napthol-As-Acetate esterase activity. Cytogenetic
analysis showed that all patients were negative for the Philadelphia
chromosome.
C. Treatment Programme
The treatment programme included three main elements: the induction
and consolidation of remission, treatment of central nervous system
(CNS) or CNS prophylaxis. and maintenance treatment.
I. Induction and Consolidation of Remission At the start of the
study we planned to give doxorubicin (adriamycin) and vincristine
every week tfor a minimum of four courses regardless of the peripheral
blood count. But the incidence of pancytopenia after the 2nd injection
was so high that the schedule was modified and the 2nd course of
doxorubicin and vincristine was given at least l4 days after the
first. The interval between the later courses of doxorubicin and
vincristine depended on the bone marrow findings.
Table I. Treatment given for inducing and consolidation
remission (OPAL a)
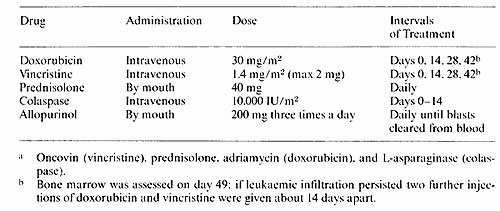
2. CNS Prophylaxis and Treatment
In the early part of the study lumbar puncture for CSF cytology
was not performed until clinical and haematological remission had
been achieved . Patients with no evidence of infiltration then proceeded
to CNS prophylaxis. This consisted of cranial irradiation (2400
rads) given in 15 fractions over three weeks with concomitant intrathecal
methotrexate 12,5 mg twice weekIv for five doses during the same
period. Analysis of the CSF findings in the first 28 patients who
achieved complete remission indicated a high incidence of asymptomatic
leukemia disease (Lister and Whitehouse, 1977). The first injection
of intrathecal methotrexate was therefore introduced during the
induction of remission, when the platelet count reached 50 X 10
high 9 /1 in the absence of circulating blast cells. The total number
of doses of methotrexate was also increased to seven. Patients with
proven CNS disease who were in clinical and haematological remission
received more intensive radiotherapy and intrathecal chemotherapy.
Cranio-spinal irradiation (2400 rads) was given in 20 fractions
together with 5 doses of intrathecal methotrexa te 12,5 mg followed
by five doses of intrathecal cytarabine 50 mg given over four weeks.
3. Maintenance Treatment
This consisted of oral 6-mercaptopurine 75 mg daily, starting when
complete remission had been achieved and always after allopurinol
had been stopped. Once CNS therapy had finished oral cyclophosphamide
300 mg weekly and oral methotrexate 30 mg weekly were started together.
The doses of all the drugs were adjusted to maintain the total white
cell count at 3 X 109/1 and treatment was continued for three years
and then stopped.
D. Cell Surface Marker Studies
The panel of membrane markers used included spontaneous sheep red
blood cell rosette formation (for T cells), reactivity with anti-human
immunoglobulin (for E cells) and with anti-ALL serum (for "common
ALL" cells). Ficol- Triosil density gradient separation of peripheral
blood or bone marrow samples was used to separate blasts and mononuclear
cells. The sheep red blood cell rosette (E-rosette) tests was performed
by addition of a suspension of 1 X 10 high 6 test cells in 50 µl
of medium with 50µl of foetal calf serum (absorbed with sheep
red blood cells) to lOO µl of a 2% suspension of sheep red
blood cells which were neuraminidase treated (15 U/ml at 37°C for
30 minutes). The cells were centrifuged at 400 g for 5 minutes and
left undisturbed at room temperature for 1 hour before gentle resuspension
and counting in an haemocytometer. The anti-immunoglobulin serum
was a fluoresceinated F(ab1 )2 preparation of sheep antibody to
human IgG (courtesy of Dr. I. Chantler, Wellcome Research ). It
was used in a direct immunofluorescence technique by incubating
1 X 10 high 6 test cells in 50 µl of medium containing 0,02%
sodium azide. with the anti-immunoglobulin at a 1 in 10 final dilution
for 30 minutes at 4 Celsisus. washing cells 3 times and counting
in suspension on a slide with a Zeiss Standard 16 phase contrast
microscope with epifluorescence and narrow band FITC filters. The
anti-ALL serum has been previously described in detail (Brown and
Capellaro. 1975). It was raised in rabbits against non- T, non-B
ALL cells coated with antilymphocyte serum. After extensive absorption
with normal haemopoietic cells. lymphocytes and acute myeloid leukemia
cells, it was functionally specific for the majority of cases of
non- T, non-E ALL and some cases of chronic myeloid leukemia in
"lymphoid" blast crisis (Roberts and Greaves, 1978). This antiserum
was used in an indirect immunofluorescence technique by incubation
for 30 minutes at 4°C with 1 X 106 viable cells in suspension, washing
cells twice and then incubation for 30 minutes at 4°C with a goat
anti-rabbit immunoglobulin antiserum which was fluorescein labelled.
The cells were then washed 2 times before counting in suspension
as above.
E Statistical Analysis
Remission duration curves and graphic presentations were developed
by standard life table formulae (Armitage 1971) and statistical
significance was determined by the Log Rank analysis method (Peto
and Pike, 1977). The significance of clinicopathological correlations
was determined by the Mann Whitney U test.
Results
A. Overall Duration of Remission
The data from 42 patients are evaluable. Twenty two have relapsed.
One elected to stop maintenance after 8 months and relapsed shortly
thereafter. He has been analysed as not having relapsed, but as
being in continuous complete remission for 8 months. One patient
died at home during an influenza epidemic whilst in complete remission.
The remainder continue in complete remission between 7 and 64 months.
The median duration of complete remission was 21 months. Seven patients
have already been in continuous remission more than 3 years.
B. Influence of Presentation Features on Remission Duration
I. Age The age of the patients at presentation did not influence
the duration of remission. The number of older patients is small,
so statistical analysis would be unwise. However, only 2 patients
out of9 over the age of40 have relapsed. both at 4 months: the remainder
continue in remission between 4 and 46 months. 2. Bulk of Disease
at presentation I. Hepatosplenomegaly (Fig. 1) Both the liver and
spleen were clinically enlarged in 20 patients. Only 6 of these
patients remained in complete remission. compared with 15 out of
22 patients in whom there was not hepatosplenomegaly. The duration
of complete remission was significantly shorter for patients with
hepatosplenomegaly (p = < .001 ). II. Blast count at presentation
All 4 patients in whom the presentation blast cell count was greater
than 100 X 10 high 9/1 had relapsed by six months. However. comparison
of the duration of remission for patients with blast cell counts
above and below 10 X 10 high 9/1 reveals no statistically significant
difference. 3. Cytochemistry The PAS reaction was positive in 20
cases and negative in 22. There was no
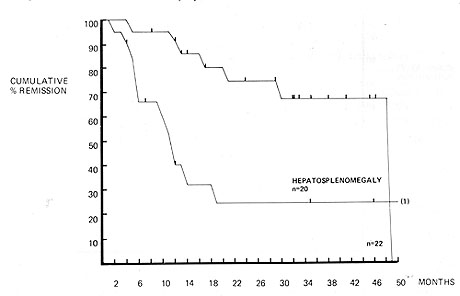
Fig. I. Duration of complete remission in acute lymphoblastic
leukemia. Influence of reactivity with anti-ALL serum
difference in the duration of complete remission between the 2
groups. Ten out of 20 patients in whom the reaction was positive
have relapsed, the remainder being in complete remission between
12 and 64 months. Eleven out of 22 patients in whom it was negative
have relapsed, the remainder being in complete remission 2 and 25
months. 4. Cell Surface Marker Studies These were performed in only
29 cases in whom complete remission was achieved. The remaining
13 cases were not studied because they were treated before the techniques
necessary were in routine use in our laboratory and not enough viable
cells were stored to allow frozen samples to be tested. Thus the
duration of follow-up of these cases is shorter than that of the
whole study and the median duration of complete remission has not
yet been reached. Complete remission was achieved in only 3 out
of 5 cases of Thy-ALL. Two have relapsed at 5 and 10 months, and
the third continues in complete remission at 35 months. The blasts
from 16 of the remaining cases of unclassified or null ALL reacted
positively with the anti-ALL serum. The duration of remission was
significantly longer than that of the 10 cases of which did not
react with the antiserum. Only 3 out of 16 anti-ALL positive common
ALL cases have relapsed. The remainder continues between 7 and 46
months. Six out of the 10 anti-ALL. non- T, non-E cases have relapsed
and only 4 remained in remission between 8 and 41 months (Fig. 2
).
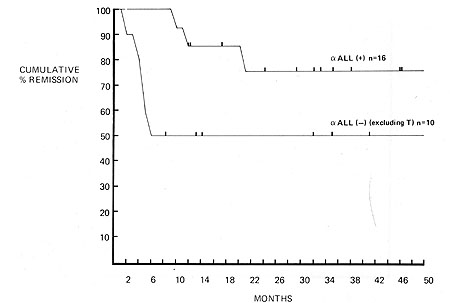
Fig.2. Duration of complete remission in acute
lymphoblastic leukemia. Influence of hepatosplenomegaly
Discussion
These results support the contention that the prognosis in ALL
in adults is influenced by the extent of disease at presentation.
The presence of hepatosplenomegaly was associated with a very short
duration of remission. All patients with a very high blast count
(greater than 100x 10 high 9/1) had relapsed within six months.
even though the previously significant adverse influence of a presentation
blast count above lOx 10 high 9/1 has not been confirmed. The cell
surface marker studies demonstrate a significant advantage for patients
whose blast cells reacted with the anti-ALL serum. The number of
patients was small and the findings should be interpreted with caution.
However. the fact that the results are identical with those reported
in childhood ALL reported by Chessels et al. Chessels and Hardisty
(1977) suggests that our observations are valid. The response to
the initial therapy remains the most important prognostic factor.
with survival being very significantly longer for patients in whom
complete remission was achieved than for those in whom it is not.
The recognition that the same prognostic factors apply to both childhood
and adult lymphoblastic leukemia makes it possible to develop treatment
programmes for adults on the basis of data obtained from childhood
studies. This is most important since the number of adults with
lymphoblastic leukemia is small and data are hard to obtain. The
recognition that presentation features influence the prognosis must
lead to the intensification of therapy for patients with adverse
prognostic factors and also the avoidance of intensification of
therapy for those patients in whom adverse prognostic factors are
not found.
References
I. Armitage. P.: Statistical Methods in Medical Research. London:
Halstead Press 1971
2. Atkinson. K.. Wells. D.G.. Clinik. H.. Kay. H. E. M.. Powles.
R.. Mc Elwain. T..J.: Adult acute leukemia. Br. .J. Cancer30, 272-278
(1974)
3. Bennett. .J. M.. Catovsky. D.. Daniel. M.- T.. Flandrin. G..
Gralnick. H. R.. Sulton. C.: Pro posals for the classiflcation of
acute leukemias. Br. .J. Haematol. 33,451-458 (1976)
4. Brown. G.. Capellaro. D.. Greaves. M. F.: Leukemia-asso(iated
antigens in man. .J. Natl. Can(er Inst. 55, 1281-1289 ( 1975)
5. Chessells. .J. M.. Hardisty. R. M.. Rapson. N. T.. Greaves. M.
F.: Acute lymphoblasti( Ieu kemia in children: Classification and
prognosis. Lancet 1977II, 1307-1309
6. Einhorn. L.H.. Meyer. S.. Bond. W.H.. Rohn. R..J.: Results of
therapy in adult acute lymphocytic leukemia. Oncology 32,214-220
( 1975 )
7. Gee. T. S.. Haghbin. M.. Dowling. M. D.. Cunningham. I.. Middleman.
M. P.. Clarkson. B.: A(ute Iymphoblastic leukemia in adults and
children. Cancer37, 1256-1264 (1976)
8. Henderson. E. S.: Treatment of acute leukemia. Semin. Hematol.
6,271-319 (1969)
9. .Jacquillat. C.. Weil. M.. Gemon. M. F.. Auclerc. G.. Loisel.
.J. P.. Delobel. .J.. Flandrin. G.. Schaison. G.. Izrael. Y.. Busel.
A.. Dresch. C.. Weisgerber. C.. Rain. D.. Tanzer. .J.. Najean. Y..
Seligmann. M.. Boiron. M.. Bernard. .J.: Combination therapy in
130 patients with acute lymphoblastic leukemia (Protocol 06 LA 66-Paris).
Cancer Res. 33, 3278-3284 (1973)
10. Lister. T. A.. Whitehouse. .J. M. A.. Beard. M. E..J.. Brearlev.
R. L.. Brown. L.. Wriglev. v c P. F. M.. Crowther. D.: Earlv central
nervous svstem involvement in adults with acute non myelogenous
leukemia. Br...J. Cancer 35,479--483 ( 1977)
11. Lister. T.A.. Whitehouse. .J.M.A.. Beard. M.E..J.. Brearlev.
R.L.. Wriglev. P.F..M.. Oliver. R.T. D.. Freeman. .J. E.. Woodruff.
R. K.. Malpas. .J. S.. Paxton. A. M..vCroowther. D.: Combination
chemotherapy f(or acute lymphoblastic leukemia in adults. Br. Med.
.J. 1, 199-203 (1978)
12.Muriel. F.S.. Pavlovsky.S.. Penalver. .J.M.. Hidalgo.G.. Benesan.
A.C.. EppingerHelft. D., De Macchi, G. H., Pavlovsky. A.: Evaluation
of induction of remission. intensification and central nervous system
prophylactic treatment in acute Iymphoblastic leukemia. Cancer 34,418-426
( 1974 )
13. Peto, R., Pike. M. C., Armitage. P.. Breslow. N. E., Cox, D.
R.. Howard, S.Y.. Mantel. S. Y., Mc Pherson. K., Peto. .J., Smith,
P.G.: Design and analysis of randomised clinical trials requiring
prolonged observation of ea(h patient. II. Analysis and examples.
Br. .J. Cancer 35, 1-39 (1977)
14. Roberts, M. M., Greaves, M. F.. .Janossy, G., Sutherland, R..
Pain. C.: Acute Lymphoblastic Leukemia (ALL) Associated Antigen
-I. Expression in Different Haemotopoietic Malignancies, Leukemia
Resear(h. 2 ( no.1) 105-114 ( 1978 )
15. Rodriguez, Y.. Hart, .J. S.. Freireich. E. .J., Bodev. GJ. P.,
M(Credie, K. B., Whitecar. .J. P.. Coltman. C. A.: POMP combination
Chemotherapy of adult acute leukemia. Cancer 32, 69-75 (1973)
16. Simone, .J.Y.. Yerzosa, M.S., Rudy, .J.A.: Initial features
and prognosis in 363 children with acute lymphocytic leukemia. Cancer36,
2099-2108 (1975)
17. Spiers. A. S. D.. Roberts, P. D., Marsh. G. W.. Paretch. S.
.J.. Franklin, A..J.. Galton. D. A. G.. Szur. Z. L.. Paul, E. A..
Husband, P.. Wiltshaw, E.: Acute Iymphoblasti( leukemia: Cy(lical
chemotherapy with 3 combinations of 4 drugs (COAP-POMP-(ART regimen).
Br. Med. .J. 4,614-617 (1975)
18. Whitecar, .J. P.. Bodey. G. P., Freirei(h, E..J.. McCredie,
K. B.. Hart, .J. S.: Cyclophosphamide (NSC -26271 ). Vincristine
(NSC-67574 ), Cvtosine Arabinoside (NSC-63878). and Predisone (NSC-10023)
(COAP) combination Chemotherapy for acute leukemia in adults. Cancer
Chemother. Rep. 56,543-550 ( 1972)
19. Willemze, R.. Hillen. H.. Hartgrink-Groeneveld, C.A.. Haanen.
C.: Treatment of acute Iymphoblasti( leukemia in adolescents and
adults: A retrospective study of 41 patients ( 1970-1973). Blood
46,823-834 ( 1975)
| 

