Prior to twenty-five years ago, there was no specific therapy for acute leukemia and survival of individuals with these diseases was usually no more than 3 or 4 months. There was no useful specific therapy, treatment consisting largely of blood transfusions and other supportive measures. X-irradiation, radioactive phosphorous, benzene, potassium arsenite, and nitrogen mustard, although of some use in chronic leukemia, were of little value in acute leukemia. Then, in 1948, Farber and his colleagues ( 1) reported that folic acid antagonists could induce com plete remission in acute lymphocytic leukemia of children.
Subsequent work demonstrated that these agents, particularly aminopterin, would induce remissions in approximately 30 % of children with acute leukemia
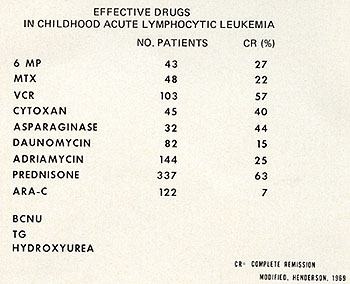
Fig. 1: Useful drugs in the treatment of childhood acute
lymphocytic leukemia. Modified from Henderson, E. S.: Treatment
of Acute Leukemia. Seminars in Hematology 6: 271-319,1969.
but in far fewer adults with acute leukemia. Unfortunately, remissions
were temporary, the patients soon became refractory to the agents
and survival was affected little, if at all. Important as these
observations were, there followed little systematic fundamental
work aimed at the control of cancer and specifically, leukemia.
However, the observations with aminopterin and amethopterin gave
rise to a good deal of optimism that curative treatment could soon
be achieved and there followed a gradually intensifying effort to
discover other drugs which could induce remissions. During the last
two decades approximately one dozen agents (Figs. 1 & 2) have been
found which are effective in acute leukemia. Some of these were
discovered empirically and others were developed as an outgrowth
of biochemical or other rationale. As a consequence, the incidence
and duration of remissions have increased greatly and survival has
gradually been extended so that median survival is now 36 months
or more for childhood acute lymphocytic leukemia (Fig. 3). In some
studies, this is now at approximately 5 years. Unfortunately, in
adult acute leukemia, progress has been far slower. Remission rates
of 50% are not unusual but survival has been lengthened only relatively
little. These results in childhood and adult leukemia have not been
achieved with any single agent but are due to the use of combinations
of drugs, abetter understanding of the importance of drug scheduling,
supportive care, and patient protection.
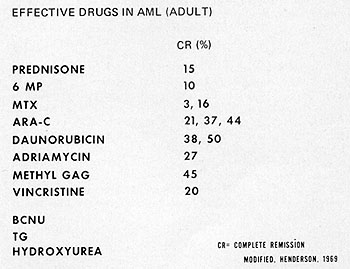
Fig. 2: Drugs for the treatment of adult acute myelocytic
leukemia. For many of these, the number of patients treated with the
individual drugs, is too few to make complete remission rates meaningful.
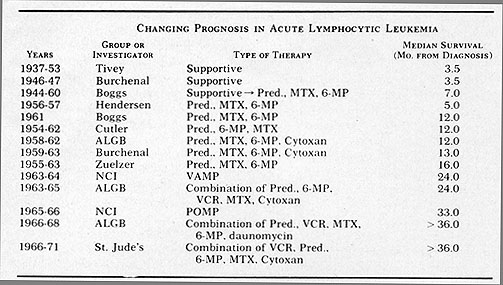
Fig.3: Progressive improvement in survival in patients
with acute lymphocytic leukemia. Carter, S. K.: The Chemotherapeutic
Approach to Cancer Therapy: A Quick Overview. In Year Book of Cancer,
1972. Clark, R. L. & Cumley, R. W. (eds). Year Book Medical Publishers,
Chicago. pgs. 475-498.
A patient with acute leukemia dies because leukemic cells have compromised
the function of an organ or normal tissue to the extent that some
vital function is no longer possible. Suppression of marrow function
is frequent either as a consequence of the disease itself or due
to the use of myelotoxic drugs. Bleeding due to thrombocytopenia
was until relatively recently the most common cause of death but
at present, infections, particularly gram negative infections, are
the most serious problem (2). The generous use of platelet transfusions
has been responsible for the diminution of fatal thrombocytopenic
hemorrhage but granulocyte transfusions have not been widely accepted
probably due to the fact that until the last few years the procurement
of normal granulocytes in large quantities has not been possible.
In addition, there were problems in designing controlled studies
to evaluate their effectiveness. However, it has recently been shown
that histocompatible granulocyte transfusions are useful in the
management of serious infections when given repeatedly to granulocytopenic
patients (3). Another approach to the control of infection has been
the use of protected environments and although their ultimate role
in cancer therapy is yet to be defined, there is strong evidence
that the incidence of infection is greatly reduced ( 4) . The strategy
in the management of patients with acute leukemia has been to attempt
to achieve rapid reduction of the leukemic cell population and restoration
of normal bone marrow function followed by therapy designed to eradicate
the neoplastic cells. Subsequently, maintenance therapy is instituted
to keep the patient in remission and prevent overt appearance of
the disease. Although with the years more agents with activity in
leukemia have been discovered, the most important factor in the
improved prognosis in acute leukemia has been the employment of
drug combinations based on the underlying principle of using agents
with different dose-limiting toxicities and with different mechanisms
of action in order to minimize the development of drug resistance.
There is abundant evidence that combinations of drugs can achieve
remission rates as great or greater than predicted for additive
effects of the single drugs employed (Fig. 4 ). The role of immunotherapy
in the management of patients with acute leukemia remains to be
determined. There is evidence for tumor associated or tumor specific
antigens on the surface of acute leukemia blast cells and prognosis
appears to be related to immune reactivity. There have been many
attempts to manipulate the immune mechanism to therapeutic advantage
using immunization with syngeneic, allogeneic or isogenic cells,
BCG and other immune enhancers, transfusion of immune sera, and
syngeneic or allogeneic bone marrow transplants. Unfortunately,
in spite of all these efforts, the role of immunotherapy in acute
leukemia remains uncertain. The success of chemotherapy in acute
leukemia is undoubtedly dependent on exploitation of differences
in cell uptake, biochemical control mechanisms, and cell kinetics
and other factors which are not completely understood. Most of the
advances in the treatment of leukemia have been achieved through
the empirical search for anti-tumor drugs. Contributing factors
include: 1. Synthesis or isolation of drugs from natural products
and their evaluation for anti-tumor activity in animal systems.
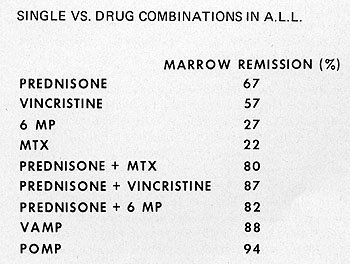
Fig. 4: Examples of superiority of drug combinations compared
to individual drugs.
2. Elucidation of their effects at the biochemical level. 3. pharmacological
and toxicological studies in animals in order to anticipate better
pharmacologic disposition and toxicity in man and to provide guidance
as to the route, dose, and schedule to be employed in man. 4. Pharmacologic
studies in man. 5. Experimental trials in cancer patients to determine
optimal dosage and schedules. Undoubtedly, one of the major factors
contributing to the success of chemotherapy, particularly against
the rapidly growing tumors has been an understanding of the importance
of drug scheduling concentrations at the target site and duration
of effect. There are now numerous examples, both experimental and
clinical, where a drug may be relatively ineffective on one schedule
of administration yet result in a total remission with prolongation
of survival on another schedule. The toxicity of an agent against
both normal and neoplastic cells is directly related to its concentration
(C) at the target and the duration of time (T) that this level is
maintained. This so-called C x T concept is markedly affected by
dose and schedules and optimally, the maximum number of tumor cells
will be destroyed with minimal effect on the normal cells. It follows,
of course, that different drugs are metabolized at different rates
and their distribution in the body may vary. Unfortunately, for
many drugs, there appears to be little correlation between schedule
dependency studies in L1210 or other experimental systems and clinical
results. One of the difficulties lies in the fact that the cellular
growth characteristics of L1210 leukemia and normal mouse marrow
and the relationship between the two does not resemble any of the
cancers in man including acute leukemia. More data are needed, not
only of pharmacologic characteristics of drugs but also of the cell
kinetics of both normal and tumor tissues at any given moment. 6.
Supportive care, as already indicated, has allowed the clinician
to treat more aggressively resulting in a greater cell kill. 7.
Appropriate therapy to eradicate sequestered leukemic cells (as
in the central nervous system). 8. The appreciation of the fact
that acute leukemia is not a single entity and that the response
to a given treatment varies according to the type of leukemia. The
traditional classification of leukemia is based on morphologic description
and clinical course and recently, cytogenetic analysis has been
added to help in identifying certain subclasses and as a guide in
prognosis. Many characteristics of leukemic cell populations -biochemical,
kinetic, colony forming, cytochemical and ultrastructural -have
been studied but most new classification proposals have been based
on the use of finer cytological characteristics than those presently
employed. Unfortunately, these are generally too difficult and controversial
for general adoption. Nevertheless, it is obvious that the current
classification is inadequate and a better scheme is needed in order
to predict the course of leukemia and response to therapy. In a
broad sense, it can be stated that the vast amount of knowledge
of leukemia including cell kinetics, biochemistry, molecular biology,
cytogenetics, virology, and immunology has had relatively little
impact on the management of patients with these diseases. This is
true in spite of many optimistic opinions often expressed by investigators
involved in these studies. The literature abounds with presumably
logical concepts of leukemia cell growth and with sequences of macromolecular
synthesis but who can say with real conviction that these reports
have as yet had any impact in changing the prognosis of even a single
patient with leukemia? It is true that within the last decade, the
relevance of cell kinetics of leukemic and normal leukocytes to
successful chemotherapy of cancer has come to be recognized. An
integral part of the anti-tumor development effort has been the
constant search for drugs with "selective toxicity"' i. e., drugs
which could selectively destroy cancer cells without undue damage
to normal cells. Unfortunately, this goal has never really been
achieved and most clinically useful agents have significant and
usually serious effects on normal tissue, particularly those with
relatively rapid turnover times, the bone marrow and the gastrointestinal
tract. As is well known, under normal circumstances granulocytopoiesis
is a cell renewal system so that cell production equals cell death.
In patients with leukemia in relapse, granulocytopoiesis usually
exceeds cell loss and an expanding cell population is the result
( 5 ). Granulocytes in the adult are produced in the bone marrow
where there is an orderly division and maturation from the earliest
cell, the stem cell, successively through the various cell types
to the mature polymorphonuclear leukocytes so that fairly distinct
morphologic compartments are identifiable (Fig. 5). In leukemia,
in contrast to the normal situation, there is evidence from cell
kinetic studies and from histological examination that leukemic
cells may be produced in a variety of sites in addition to the bone
marrow, i. e., lymph nodes, liver, spleen, testes, etc. The process
of maturation and differentiation is disturbed and morphologic classification
based on maturation is usually not possible. Available evidence
suggests that a cell perhaps similar to a small lymphocyte may be
the common stem cell and that the erythroid, myeloid and megakaryocytic
cell lines are probably derived from this pluri-potential cell.
The stem cell compartment must be able to maintain itself against
continued removal of cells for differentiation, reconstitute itself
if depletion occurs, and be capable of increasing its rate of cell
production upon demand. There is now good evidence in man that there
is a single compartment which gives rise to these various cell lines.
Support for this concept is provided from the observations by Whang
et al. that the ph 1 chromosome is present not only in granulocyte
precursors but also in erythrocytic and megakaryocytic precursors
( 6 ). This suggests that the chromosomal defect arises in a cell
which is a common stem cell for the three cell lines.
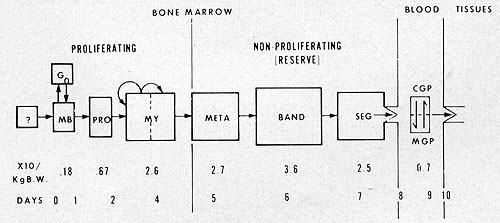
Fig. 5: Model for normalleukocyte kinetics.
Similarly, studies of the hematopoietic system of the mouse utilizing
the spleen colony technique have also provided data suggesting that
there is a single pluripotential stem cell. In addition to the stem
cell compartment there is also a large differential proliferating
pool consisting of myeloblasts and promyelocytes. The next compartment
in the sequence is the myelocyte pool composed of large and small
myelocytes; the large cells representing a dividing pool supplying
cells to the small cell maturation pool. The proportion of proliferative
cells in the bone marrow of patients with acute leukemia is relatively
low compared to normal marrow (7-10). In normal bone marrow, approximately
one-third of the myeloid cells are in proliferation with an average
labeling index of abou t 30 % ( 11) .Generation times for the myeloblasts,
promyelocyte, and myelocyte have been estimated at 24, 60, and 54
hours respectively (12) with a maximum DNA synthesis time of 24
hours. It is now known that there may be a wide distribution of
intervals for each of the phases. The variability in length of the
G1 phase has the most relevance to the chemotherapyofpatients with
leukemia since most of the presently available anti-leukemic agents
do not affect cells in the long G1 or so-called Go phase. This will
be considered at greater length below. With the completion of maturation,
the granulocyte enters the so-called "mature granulocyte reserve"
of the bone marrow. Estimates vary, but there are approximately
2-3 x 1011 granulocytes in this compartment (13), and there are
thus 10-20 times as many bands and segmented granulocytes in reserve
as there are circulating in the blood. The release of granulocytes
into the blood is an interesting phenomenon which unfortunately
is not well understood. Recent work suggests that changes in the
biophysical properties of the cytoplasm as differentiation and maturation
occur ma y be important factors ( 14) . It is important at this
point to mention, if only briefly, some of the observations which
have been made in recent years concerning granulocyte production
in vitra (15). With both mouse and human bone marrow cells, colonies
grown in vitra and arising from the colony-forming cell (CFC) require
the continuous presence of a stimulatory substance, colony stimulating
factor (CSF), which is found in sera and urine from normal and leukemic
individuals and from mice. In the absence of this material, colony
growth is not sustained and the cells rapidly die. It has been suggested
that CSF is specific for neutrophils and that its major source are
mature granulocytes. If this were the case, there would be no stimulus
if an individual were rendered neutropenic and increasing myelopoiesis
would result in the presence of granulocytosis. To confuse the issue
further, there is good evidence that mature granulocytes are inhibitory
(16) and that monocytes may be the source of material controlling
granulocytosis (17). CSF is a glycoprotein with a molecular weight
of approximately 190,000 and is considered by many to be a growth
regulator or granulopoietin for the granulocytic series analogous
to erythropoietin for the red cell series. The function of CSF in
viva has not yet been elucidated; however, patients with acute lymphocytic
or stem cell leukemia generally have elevated levels while those
with acute myelocytic leukemia have depressed levels (18). During
remission, the levels in patients with acute myelocytic leukemia
rise to normal or high values. Diffusible granulocytopoietic stimulator
(DGS) has been reported to be present in viva in mice following
the injection of endotoxin or after irradiation and has been shown
to stimulate granulocyte production in Millipore filters implanted
intraperitoneally ( 19 ). Preliminary data suggest that this material
is different from CSF. The relationship of CSF, DGS, chalones and
other inhibitors, antichalone and leukocyte inducing factor is at
present unclear and certainly somewhat bewildering. If there is
a defect in this system in leukemia, its precise location is difficult
to ascertain from reports in the literature. Finally, the significance,
if any, of these observations for the treatment of patients with
leukemia remains to be determined. In contrast to the orderly unidirectional
progression of division, maturation and release from the bone marrow
of leukocytes in the hematologically normal individual, the picture
in leukemia is largely one of confusion with marked deviation from
the steady state (Fig. 6). In acute leukemia, normal leukocytes
are replaced by large numbers of blasts both in the bone marrow
and in the peripheral blood where the count mayor may not be elevated.
The spleen, liver and lymph nodes may be infiltrated with these
cells and enlarged. Years ago, it was assumed that in leukemia the
orderly process of normal myelopoiesis was greatly disturbed owing
to some unidentified influence and the myeloid precursors were rapidly
and excessively proliferating. This hypothesis was never substantiated
and was replaced by the current concept, first suggested by Astaldi
and Mauri (7) that leukemic cells do not proliferate wildly, but
that there is some maturation defect accompanied by the accumulation
of large numbers of immature myeloid cells.
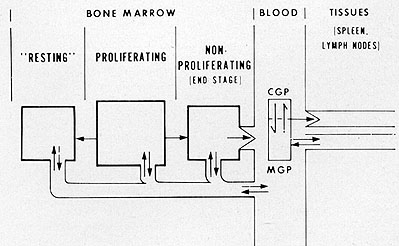
Fig. 6: Model for leukocyte kinetics in leukemia.
Based on stathmokinetic and in vitra labeling studies with ³HTdR,
Gavosto et al (8,9), suggested that the proliferative capacity in
acute leukemia was very low compared to normal bone marrow and that
the labeling index of blast cells in acute leukemia was in proportion
to the size of the cells, the larger cells being considered the
younger ones. These cells, in both AML and ALL, com prised a relatively
small percentage of leukemia cells in the bone marrow and had a
high labeling index (range 24-52) both after in vitra labeling with
³HTdR and after a pulse label in viva (20). In contrast, the labeling
index of the small cells was quite low. It is now generally accepted
that the large cells are the dividing or cycling population and
that the small cells are the "resting" (Go) or non-proliferating
population. However, this population is obviously not "resting"
in the strict sense and most likely is comprised of cells in a very
prolonged G1 phase. It is hypothesized, based on the interpretation
of data obtained in patients with acute leukemia using ³HTdR labeling
(21), that the small "non-dividing" leukemia cells are capable of
re-entering the proliferative cycle. Studies in the spontaneous
AKR mouse leukemia employing a cell separation technique conclusively
demonstrate that the small cells have a normal component of DNA
and even after labeling with 3 HTdR for a period equivalent to 5
cell cycle times, unlabeled cells are still present. These small
cells are heterogeneous consisting of both non-clonogenic cells
and clonogenic cells residing in either a Go or along G1 phase of
the cell cycle (22-24 ). Upon transplantation to young normal AKR
mice, the small cells are capable of proliferating and causing death
due to leukemia. There have been many cell kinetic studies in acute
leukemia and although some of the data on cell cycle characteristics
of leukemic leukocytes may be suspect it appears that (1) the majority
of leukemic cells are capable of DNA synthesis but that most of
the blasts are not in active proliferation ( 2) cell cycle times
vary greatly (25-28), ranging from 60 to 200 hours and are generally
somewhat longer than those for the early normal myeloid precursors
and (3) the intravascular life of leukemic leukocytes is prolonged.
In contrast to the simple exponential intravascular disappearance
pattern of normal granulocytes, leukocyte disappearance curves in
patients with acute leukemia are often complex and prolonged (29-30).
This may be present even when the patients are in remission and
suggests that morphologically normal appearing granulocytes in these
patients are still defective. On the other hand, extra-corpuscular
factors cannot be ruled out since prolonged intravascular curves
have been reported in patients with non-leukemic malignancies (31).
In hematologically normal individuals, granulocytes once having
left the vascular tree, do not return but in AML (32,33) as in CML
(34) leukemic cells may enter the spleen and then recycle to the
blood and the bone marrow. Leukemic cells are rarely seen dividing
in the peripheral blood and the proportion able to incorporate 3HTdR
is less than that in the bone marrow. The foregoing is a brief review
of the current status of information concerning leukocyte kinetics
in acute leukemia. The precise defect in acute leukemia specifically
acute myelocytic leukemia, is not known but as has been postulated
by Gallo (35) and others, the findings are consistent with a block
in the normal process of maturation of myeloid elements. Until the
in vitro colony work discussed above this was considered irreversible
but it now appears that leukemic cells can be made to mature under
appropriate circumstances in the presence of a certain protein factor(s).
The cause of this disturbance in maturation is also not clear at
the present time but in the last two or three years, a great deal
of evidence has been accumulated strongly suggesting that RNA tumor
viruses are involved. It is beyond the scope of this paper to review
this evidence but regardless of whether one accepts the oncogene
theory or the protovirus theory, the finding of the enzyme, reverse
transcriptase, may be a most important development as far as the
potential for controlling or curing acute leukemia. This enzyme
appears to be distinct from RNA dependent DNA polymerase activities
which have been reported in normal cells (36, 37). If reverse transcriptase
is uniqqe to leukemic cells it represents a prime target for therapeutic
attack providing its presence is required for maintenance of the
neoplastic state. Other DNA polymerases in leukemic cells, if qualitatively
different from their counterparts may also be important targets.
In any case, the reports of selective toxicity of rifamycin derivatives
for leukemic cells are exciting ( 38) even though the precise mechanism
for this toxicity is still unclear (37). Undoubtedly, other compounds
will be found with similar or better selectivity. The accumulating
evidence suggesting that a virus may be the etiologic agent in leukemia
and that reverse transcriptase plays an important role in the initiation
of the desease and perhaps, in its maintenance raise important questions
particularly in relation to relapses in patients after long apparently
disease free intervals. Such relapses have been postulated to be
due to 1) persistance of resting cells and their re-entry into cycle
2) a failure of the immune mechanism in preventing the appearance
of clinically detectable leukemic cells arising from a small cluster
of cycling cells 3) re-induction by the agent responsible for the
initial even t. The latter possibility gains some support from the
experience with normal marrow transplants into leukemic patients
in which leukemic transformation of donor cells were observed. However,
other explanations for this phenomenon are possible. In addition,
specific cytogenetic abnormalities when present in acute leukemia
tend to disappear when the patient is in remission but the same
abnormalities recur in late relapses. It would be most unlikely
that a virus would cause precisely the same abnormality upon re-infection.
However, it is conceivable that a sub-virus moiety might bind at
the same site and produce the same karyotypic defect. How has this
knowledge I have reviewed been utilized in the management of patients
and has it been useful ? Based on data from animal studies and certain
kinetic considerations it is possible to conceptualize ( Fig. 7)
neoplastic cell populations, including leukemia (20). Populations
with a high proportion of cells in active proliferation and with
a high clonogenic potential are classified into compartment A; cells
temporarily non-dividing but capable of re-entering the growth cycle
( cells in Go or in with a prolonged Gl phase) are in compartment
B; cells which are incapable of reverting to proliferation and are
end-stage or mature are in compartment c; and finally, dying cells
and cells undergoing lysis and resorption belong to compartment
D. In leukemia and in other neoplastic populations, growth occurs
when the input from compartment A exceeds the loss in compartment
D ( or with A constant, the loss in D decreases, a very unusual
situation). At an early stage, the proportion of cells in active
cycle (i. e. in A) is high and the proportion in a resting phase
(i. e. in B) is low. As the disease progresses, the proportion in
B increases and the doubling time of the whole population lengthens.
This change in proliferative characteristics from early exponential
growth is best described by a Gompertzian function (39). Obviously,
the deviation from exponential growth may also occur from an increase
in cell loss, a lengthening of Tc, or a combination of these factors.
It is important to note, however, that growth fraction and cell
loss are probably the prime determinants governing the rate of tumor
growth although growth characteristics may be changed as a consequence
of therapy. I t has been shown, in fact, that regrowth of L1210
following treatment with BCNU is accompanied by cells dividing with
a longer Tc ( 40) and similar observations have also been reported
in acute leukemia (41). Remissions occur when the loss in compartment
D exceeds the input from A. Most clinical by useful anti-tumor drugs
affect compartment A cells. These are the socalled cycle active
drugs and include the anti-metabolites and the mitotic inhibitors.
Alkylating agents and functionally related compounds probably have
their major effect on compartment A cells but do also exert an effect
on cells in compartment B. Unfortunately, the effects on these cells
are not well understood and as will be seen, the persistence of
these cells after treatment represents one of the serious problems
in the management of patients with leukemia. Kinetic data on normal
and leukemic animal and human leukocyte populations have been examined
with relation to response to chemotherapy (20). A number of observations
emerge including 1) there is a direct relationship between the labeling
index and the response to chemotherapy; 2) there is an inverse relationship
between doubling time and response and 3) alkylating agents are
more effective against tumors with long doubling times and low growth
fractions ( compartment B) compared to anti-metabolites.
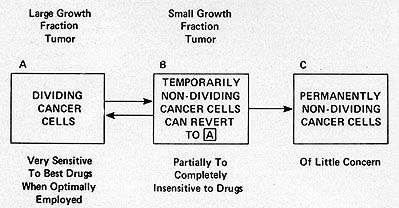
Fig. 7: Relationship between tumor growth characteristics
and response to therapy ( modified from ref. 20)
Responsiveness appears to be related to the size of the growth fraction
since as the growth fraction decreases with advancing disease, the
likelihood of obtaining a tumor regression or cure declines. At
diagnosis, in an adult with acute leukemia, there are approximately
10 high 12 leukemic cells and the labeling index is quite low. A
"remission" by current criteria is achieved when a 3 log reduction
in cells is obtained with chemotherapy and although there may be
10 high 9 leukemic cells in the body, they are not detectable by
the presently available techniques. Experience has shown, however,
that continued aggressive therapy is necessary or the patient will
quickly relapse. In any case, even though normal myeloid precursors
are also affected by the agents employed, the normal elements regenerate
more rapidly (shorter cell cycle time and higher growth fraction)
and the leukemic cells are no longer detectable on blood or bone
marrow examination. If the body burden in acute leukemia at diagnosis
or relapse totals approximately 10 high 12, theoretically a 12 or
13 log reduction should affect a cure. The word "theoretically"
needs to be emphasized since it may not be necessary to achieve
at 12 or 13 log reduction for a cure if the immune mechanism is
invoked to eradicate the last 2 or 3 logs of tumor cells. In most
cases of acute lymphocytic leukemia, the most responsive of the
acute leukemias, the body burden of leukemic cells is reduced to
10³ or 10 high 4 cells following vigorous combination chemotherapy.
With prolonged therapy there is evidence that residual leukemic
cells may number lOO or fewer and there are data in both man (25)
and animals (23) that these remaining cells may be predominantly
resting cells. Following continuous infusion of ³HTdR in patients
with acute leukemia for as long as 20 days, a small but significant
proportion of leukemic cells remain unlabeled (25). In spontaneous
AKR leukemia, as discussed above, these small cells upon transplantation
to young normal AKR mice, are capable of proliferating and causing
death due to leukemia ( 22 ) .There is good reason to believe that
the kinetic behavior of leukemic cells in the advanced disease in
man is similar to that of the leukemic population in spontaneous
AKR leukemia and it appears quite likely that resting small cells
in the human disease are also capable of resuming proliferation.
Since resting cells are relatively insensitive to current chemotherapeutic
agents, it would appear to be appropriate to use some form of immunotherapy
in an attempt to eradicate them completely. However, thus far, as
discussed above, this has not be achieved. Both advanced L1210 leukemia
and spontaneous AKR leukemia are relatively insensitive to cycle
active agents, presumably due to the low growth fraction in both
situations. However, if the total leukemic cell population is reduced
by treating with a non-cycle active drug, the residual cells are
stimulated to resume proliferation and are then susceptible to a
cycle active agent such as arabinosylcytosine (42,43). This concept
underlies some of the attempts to gain a therapeutic advantage in
human leukemia. For example, extracorporeal irradiation ( 44 ),
intensive leukapheresis ( 45) and attempts at cell synchronization
( 46) have been employed in an effort to recruit resting cells to
enter proliferation in AML. Unfortunately, these procedures have
not lead to a higher remission rate or to a prolongation of survival
following treatment. It is obvious that elucidation of the control
mechanisms governing both the entry of cells into prolonged G1 or
Go is urgently needed. A great deal of consideration in this paper
has been given to attempts to achieve selective toxicity for tumor
cells by trying to take advantage of a variety of differences between
normal a1nd neoplastic cells such as growth characteristics. Although
these have not been totally successful, important progress has been
achieved in controlling cancer in man. However, there are other
avenues which deserve important emphasis and some of these, particularly
following on the recent developments in molecular biology, have
already been mentioned. Another approach which deserves attention
lies in studies of the cell membrane. There is growing evidence
that neoplastic cell surfaces may have therapeutically exploitable
differences. The work with concanavalin A and wheat germ agglutinin
has helped to elucidate cell membrane structure ( 47 , 48 ). The
agglutination of viral and chemically transformed cells is of great
interest although some normal cells are also affected (49). These
observations appear to deserve further work for potential application
to treatment of patients with leukemia and other neoplastic disorders.
In summary, in this paper, I have attempted to review some of the
concepts in acute leukemia and the status of treatment of patients
with these diseases. Recent developments in several areas directly
and indirectly related to leukemia add greatly to our knowledge
of these disturbances and appear to have important implications
for their control or cure.
References
1. Farber, S., et al. (1948) N. Eng. J. Med. 238,787-793.
2. Perry , S. ( 1971) Cancer Chemotherapy Reports. 2, 99-104.
3. Graw, R. G., et al. (1972) N. Eng. J. Med. 287,367-371.
4. Levine, A. S., et al. (1973) N. Eng. J. of Med. 288,477-483.
5. Perry, S. (1971) Annual Rev. of Med. 22,171-184.
6. Whang, J., et al. (1963) Blood. 22,664-673.
7. Astaldi, G., and Mauri, C. (1953) Rev. BeIge Path., 23, 69-82.
8. Gavosto, F., et al. (1960) Nature. 187,611-612.
9. Gavosto, F ., et al. (1964) Nature. 203, 92-94.
10. Mauer, A. M., et al. (1966) Blood. 28, 428-445.
11. Killmann, S. A. (1968) Ser. Haemat. 1, 38-102.
12. Cronkite, E. P., et al. (1964) New Eng. J. of Med. 270, 1347-1352,
1403-1408.
13. Perry, S., et al. (1966) J. Clin. Invest. 45,1388-1399.
14. Lichtman, M. A. (1970) N. Eng. J. Med. 283, 943-948.
15. Metcalf, D. and Moore, M. A. S. (1971) Med. J. Aust. 2,739-746.
16. Paran, M., et al. (1969) P. N. A. S. 62,81-87.
17. Chervenick, P. A. and Lo Buglio, A. F. (1972) Science. 178,164-166.
18. Robinson, W. A. and Pike, B. L. (1970). N. Eng. J. Med. 282,1291-1297.
19. Rothstein, G., et al. (1973) Blood. 41,73-78.
20. Skipper, H. E. and Perry, S. (1970) Cancer Res. 30,1883-1897.
21. Saunders, E. F. and Mauer, A. M. (1969) J. Clin. Invest. 48,
1299-1305.
22. Rosen, P. J ., et al. (1970) JNCI, 45, 1169-1178.
23. Omine, M. and Perry, S. (1972) JNCI, 48,697-704.
24. Omine, M. and Perry, S. (1973) Cancer Res. 33, 2596-2602.
25. Clarkson, B. D. (1969) NCI Monograph 30, Human Tumor Cell Kinetics.
81-120.
26. Saunders, E. F. (1967) J. Clin. Invest. 46,1356-1363.
27. Clarkson, B., et al. (1967) J. Clin. Invest. 46,506-529.
28. Gavosto, F., et al. (1967) Nature. 216,188-189.
29. Spivak, J. L., et al. (1969) Blood. 34,582-590.
30. Galbraith, P. R., et al. (1970) Blood. 36,371-384.
31. Galbraith, P. R., et al. (1965) Blood. 25,683-692.
32. Rosen, P. J. and Perry, S. (unpublished).
33. Killmann, S. A., et al. (1971) Acta. Med. Scand. 189,137-142.
34. Moxley, J. H., et al. (1965) Nature. 208,1281-1282.
35. Perry , S. and Gallo, R. C. (1970) A. S. Gordon, Ed Appleton-Century-Crofts,
New York, Vol. II. pp. 1221-1272.
36. Gallo, R. C., et al. Proceedings of the Second Annual Steenbock
Symposium. (1973) Eds: Wells, R., Duman, R., Univ. Park Press, Baltimore
252-286.
37. Gallo, R. C., et al. Proceedings of the Fifth International
Congress of pharma cology, S. Karger, Basel (1973) 3,411-436.
38. Smith. R. G., et al. (1972) Nature New Biology. 236, 166-171.
39. Laird, A. K. (1965) Brit. J. Cancer, 19,278-291.
40. Young, R. C. and De Vita, V. T. (1970) Cancer Res. 20,1789-1794.
41. Clarkson, B. D., et al. (1970) Cancer. 25, 1237-1260.
42. Skipper, H. E., et al. (1969) Cancer Chemo. Repts. 53,345-366.
43. Tyrer, D. D., et al. (1967) Cancer Res. 27, 873-879.
44. Chan, B. W. B. and Hayhoe, F. G. J., (1971) Blood. 37,657-663.
45. Peich, L., et al. (1971) Proc. AACR. 12,25.
46. Lampkin, B. C. (1972) Seminars in Hematology. 9,211-223.
47. Burger, M. and Noonan, K. (1970) Nature. 228, 502-503.
48. Burger, M. and Goldberg, A. (1967) P. N. A. S. 57,359-366.
49. Inbar, M. and Sachs, L. (1969) P. N. A. S. 63,1418-1425.
|






