|
Beckman Research Institute of The City of Hope 1450 East Duarte Road Duarte, CA 91010 I pay homage to Umberto Eco who attended this meeting by quoting
the very first sentence of PROlOGUE to his widely acclaimed book
"THE NAME OF THE ROSE" in English translation: "In the beginning
was the Word and the Word was with God and
the Word was God". It - would be noted that in this
17-word-long sentence, the word recurrs thrice and God
twice; all together, recurring words occupying half of the sentence.
Indeed, the essence of any good writing appears to depend upon the
recurrence at more or less regular intervals of the same or similar
sounding words which give a lyrical quality to it. Julius Caesar's
announcement to the Roman senate of his victory at Zela (47 B.C.)
survives to this day, only because all three word-combinations uttered
in succession began with v and ended in i; "ve'ni, vi'di, vi'ci".
This then is the extreme of recurrence with no interval at all.
In immunology, the antigen-specific cooperation between helper T-cells
and antibody-producing B cells appears again to depend upon recurrence
of the same word (the same signal ). As schema tically illustrated
in Figure 1, a macrophage phagocytizes antigen "A" and digests it
to a number of small peptide fragments. Of those digested fragments,
an amphipathic alfa-helical fragment is preferentially chosen and
presented to the outside world by the antigen-presenting macrophage
in conjunction with the self class II MHC antigen (extreme left
of Figure 1). A clone of T-cells which happen to possess the membrane-bound
receptor that fits this antigen "A" alfa-helical fragment + self
class II MHC antigen complex, now becomes antigen-specific helper
T-cells (middle of Figure 1). But how this clone of T-cells can
selectively recognize and help not a single clone but a number of
clones of B-cells equipped with membrane-bound antiantigen "A" anti-bodies
of IgM and IgD types? For it would be recalled that antibodies are
constructed to recognize antigens per Be not complexed with self
class II MHC antigen. Further more. each polypeptide antigen usually
present not one but a number of antigenic determinants. Accordingly.
there ought to be and are several different clones of B-cells; their
antibodies being directed against different antigenic determinants
of antigen "A". Nevertheless. having been endowed with membrane-bound
anti-antigen "A" antibodies. all these different clones of Bcells
would manage to concentrate antigen "A" on their cell surface. These
antigen-antibody complexes shall be lysostripped off the plasma
membrane and shall be digested inside B-cells. As the digestive
enzyme involved is of the same sort as that present in the macrophage.
antigen "A" is digested to the same variety of peptide fragments
and the same amphipathic alfa-helical fragment is chosen and presented
by B-cells to the outside world in conjunction with self class II
MHC antigen. It is this complex which the receptor of helper T-cells
recognizes. thus. resulting in antigen-specific help to expand all
clones of antiantigen "A" B-cells (extreme right of Figure I). 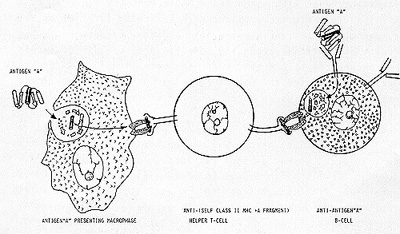
All in all. it would thus appear that the antigen-specific cooperation between T-cells and B-cells is based upon one principle; that when confronted with the same sentence (antigen "A"). both antigen-presenting macrophage and anti-antigen "A" B-cells chose the same word (a particular amphipathic alfa-helical segment) out of that sentence and crowns it with the same adjective (self class II MHC antigen).
In immunology. one often speaks of specific antigen-antibody interactions as examples of recognition based upon the complementarity between two components. Nevertheless, the fact is that all components of the adaptive immune system are composed of strings of repeating units ultimately derived from the common ancestral unit. This unit commonly referred to as the ▀2-rnicroglobulin-like domain is made of 90 to 100 mostly hydrophobic amino acid residues, the relative abundance of hydrophilic Ser and/or Thr also being a conspicuous feature. These residues are folded into three to five loops of anti-parallel ▀-sheet forming segments. Contacts between neighboring ▀-sheet forming segments are maintained through hydrogen bonds mostly formed between Thr-Thr, Thr-Ser or Ser-Ser, and the whole structure is compacted by the presence of one intradomain disulfide bridge. It now appears that the immediate ancestor of genes for the adaptive immune system was CAM (cell adhesive molecule) gene engaged in organogenesis of early embryos. In the extracellular portion of N-CAM specific for neuronal organization, four successive ▀2-rnicroglobulin-like domains were found (Hemperly et al. ,1986). Through these domains, N-CAM engages in homologous recognition, thus, aggregating similar neuronal cells; the first step in neuronal organization. It is fitting that all components of the adaptive immune system evolved from CAM; the original mediator of cell-cell interaction. The point to be made here is that the ▀ 2-rnicroglobulin-like domain originally evolved to engage in homologous, not complementary, recognition. Accordingly, recognition of class I and Class II MHC antigens by T-cell receptors and 8-cell antibodies, as well as that of idiotypes by another T-cell receptors and B-cell antibodies are homologous recognition sensu stricto; the notion of complementary recognition being more of an illusion than reality
Implicit in the above stated notion that the immediate ancestor
of various components of the adaptive immune system was one of the
cell adhesion molecules (CAM) involved in the initial stage of embryonic
organogenesis is the assumption that four ▀2-rnicroglobulin-like
domains of N-CAM arose in situ. Were they borrow ed from other molecules
(even from immunoglobulins themselves) by the so-called domain exchange,
the whole notion of CAM being the immediate ancestor of various
components of the adaptive immune system becomes ridiculous. Fortunately,
it looks as though these ▀2-microglobulin-like domains of N-CAM
indeed evolved in situ, for there is a noticeable similarity in
construction of these ▀2-microglobuin-like domains and other parts
of N-CAM. Each of these ▀2-microglobulin-like domains contains three
absolutely invariant residues; 1) Cys in 12th position, 2) Trp in
24th position, and 3) Cys in 62nd position. These three invariant
residues tend to be included in Thr-X,Thr-X dipeptidic repeats.
This is illustrated at the top of Figure 2 on 3rd of the four success.
ive ▀2-microglobulin-like domains, for 3rd is the only complete
domain, the other three sustaining deletions of three to six res.
idues (Hemperly et al. ,1986). Four successive ▀2-microglobulin
like domains comprise but 40% of N-CAM polypeptide chain. The 362-residue
long carboxyl terminal domain remaining within the cell is constructed
of a simpler mode, thus, suggesting that this segment remained close
to the original design of the entire CAM polypeptide chain. As also
shown at the top of Figure 2, 699th to 728th residues of N-CAM is
esentially made of Thr-X, Thr-X dipeptide repeats. Thus, it is conceivable
that the entire coding sequence for the ancestral CAM was simple
repeats of something like ACT C C A A, ▀2-microglobulin-like domains
too evolving from parts of it. Three consecutive copies of sucl
a heptamer, 21 bases in the total length would have given the heptapeptidic
periodicity to the original peptide chain as showl below:  Two base substitutions affecting the above noted periodicity uni' would have produced three consecutive Thr-X dipeptides as show below; two substituted bases are underlined:  At the top of Figure 2, ACT C portion of this hypothetical heptameric unit and its single base substituted deviants are solidly underlined. Is there any validity to the above noted proposal as to the ulti mate origin of CAM coding sequences. The first CAM must have come into being when the first multicellular eukaryote evolved from unicellular eukaryotes. Slime molds of the genus DictyOs telium indeed occupies a unique position of being an intermediate between unicellular and multicellular eukaryotes, for these organisms in nutrient rich environments live as unicellular 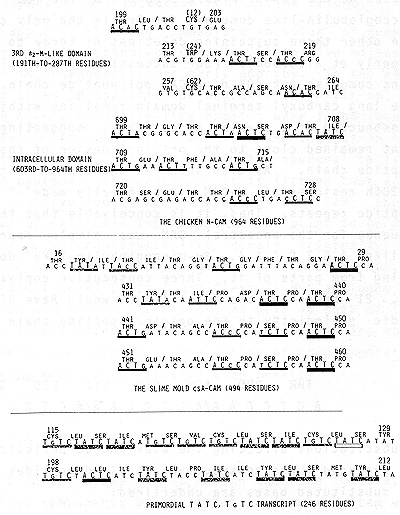
In our galaxy and others, stars have been formed and are still being formed by gravitational condensation of molecular clouds that contain large quantities of molecular hydrogen, water, ammmonia, carbon monoxide, methyl alcohol, hydrocyanic acid and others. When the earth was formed some 4.5 billion years ago, the primeval atmosphere surrounding it must have also contained these chemically reducing compounds noted above (Holye,1979; Dyson, 1985). In the classical experiment of Miller in 1953, electric sparks passed through a mixture of methane, ammonia, molecular hydrogen and water yielded large fractions of amino acids; notable being alanine of a 2% yield. Oro in 1960, on the other hand, prepared a concentrated solution of ammonium cyanidein water. After a period, he found spontaneous converison of ammonium cyanide to adenine with 0.5% yield, (Miller and Orgel, 1974). Thus, it might be said that the yielding of various building materials of life was and is inherent in the composition of molecular clouds. What is life but a form that reproduces near exact replicas of itself. Thus, we owe our lives to the inherent complementarity that exists between the two purinepyrimidine pairs of bases. Adenine pairs with uracil or thymine, while guanine forms hydrogen bonds with cytosine. Accordingly, when two complementary strand of double-stranded nucleic acids fall asunder, each can form its complementary strands. By this way, nucleic acids are inherently designed to perpetuate their base sequences. Inasmuch as the copying of the template, that is to say building of a new single stranded RNA complementary to the preexisted single stranded RNA is based upon the above noted inherent complementarity between A and U as well as G and C, this could have taken place in the prebiotic world, for if provided with a template as long as 60 to 100-base-long, AT P, G T P, UT P and C T P would align themselves in the proper 3'-5' linkage to form a complementary strand in the presence of Zn++ metal ion alone (Bridson and Orgel,1980). The major obstacle in the prebiotic world against spontaneous generation of the first cell on this earth, thus, was the formation of long enough templates directly from AT P, G T P, UT P and C T P, for even in the presence of imidazol and Zn++, autopolymelization of nucleotide triphosphates yields only base hexamers to decamers. It follows then that unless these base oligomers were endowed with the inherent property for self elongation, long enough templates would not have come into being to start life on this earth. What if a given base octamer was repeats of the base tetramer such as TAT C already noted This octamer and its complementary strand formed after the first round of copying may have reannealed unequally first copy to the second copy after falling asunder as illustrated below: T A T C T A T C The hydrogen bonded paired portion would have served as a primer
for the next round of copying (replication), and after this round,
the octameric template would have elongated itself to the dodecameric
template. Indeed, self elongation is inherent in repeats of base
oligomers (prebiotic nucleic acids were RNA rather than DNA, thus,
two T's of TAT C should have been substituted by U's, but for the
sake of continuity, U AU C is shown as TAT C). This, then, is one
of the many reasons for believing that the first set of coding sequences
emerged at the very beginning of life on this earth were all repeats
of base oligomers (Ohno and Epplen,1983). Indeed, we have already
seen that mixed repeats of TAT C and its single base substituted
deviant T G T C appear to have served as the ultimate ancestor of
one superfamily of genes; first various CAM's for general cell-cell
recognition during the initial stage of organogenesis of all multicellular
eukaryotes and through them, various components of the adaptive
immune system unique to vertebrates. It would be noted that such
tetrameric repeats resemble Julius Caesar's remark already cited
in construction. vi'di in the middle can be considered as TAT C,
then ve'ni preceeding it becomes its two base substituted copy such
as T G A C, while vi'ci following it becomes its single base substituted
copy such as T G C C. Such tetrameric repeats also resemble musical
compositions of the Baroque period. As an example, the treble clef
musical score of Prelude No.1 for well-tempered clavichord by Johann
Sebastian Bach (16856-1750) is shown in Figure 3. It would be noted
that the initial part of this treble clef score in C major (the
top 2 and 2/3rd lines of Figure 3) is essentially four note repeats;
the second half of each 8/8th time signature segment being the exact
copy of the first half. Each half of the time signature segment
is comprised of two sets of the identical four notes; 4th note of
the 1st set overlapping with 1st note of 2nd set. From the last
one-third of the 3rd line of Figure 3 and downward, the theme now
changes to three note repeats. This is because 1st note of each
previous four note unit is now relegated to the base clef score.
Such striking resemblance between Baroque musical compositions and
primordial coding sequences that are repeats of base oligomers tempted
us to devise one invariant rule by which treble clef scores of musical
compositions and coding base sequences become interchangeable. After
considering their respective molecular weights and complementarity,
we have decided to assign a space and a line above it of the treble
clef staff to each of the four bases in the ascending order of A
G T C; Con the line of the previous scale occupying the classical
middle C position (Ohno and Ohno, 1986). This assignment of bases
to the treble clef staff afforded a needed freedom in transmutating
coding base sequences to treble clef musical scores. This freedom
is analogous to that accorded to coding sequences by the redundancy
of  Figure 3 The treble clef score of an initial portion of J.S. Bach┤s Prelude No.1 from well-tempered clavichord is shown accompanied by a base sequence transcribed from it according to the previously devised invariant rule (Ohno and Ohno,1986). Initial tetrameric repeat portion should have encoded a polypeptide chain of tetrapeptidic periodicities, except for an unfortunate concentration of chain terminaters T A A's and T A G's at the extreme right of 2nd line. Subsequently, the treble clef score becomes trimeric repeats monotonously encoding homoserines occasionally interspersed by stretches of homoisoleucines and homoarginines. 

 Figure 4, Part III
1. Bridson, P.K, and Orgel, L.E. (1980) Catalysis of accurate
poly (C) directed synthesis of 3'-5' linked oligoguanytes by Zn+2.
J. Mol. Biol. 144:567-577. |