|
MRC Molecular Haematology Unit, Institute of Molecular
Medicine John Radcliffe Hospital Headington, Oxford. OX3 9DU, UK
* to whom correspondence should be addressed
2 Laboratoire de Biologie Cellulaire et Moleculaire des Facteurs
de Croissance, CNRS, Villejuif, France
3 Division of Hematologic Malignancies, Dana-Farber Cancer Institute,
Harvard Medical School, Boston, USA
4 Institute of Child Health, London, UK 5 Institute of Cancer Research,
London, UK.
Introduction
This European concerted action was established to promote collaborations
and the exchange of information amongst European laboratories working
on human haemopoietic stem cells. It is supported for three years
by the European Commission, Directorate General XII under the direction
of Dr Ch. Bardoux. There are, at present, over 56 contributing laboratories
throughout Europe with 2 laboratories in Central and Eastern Europe
joining through the PECO initiative in late 1994. The co-ordinator
[Dr. S.M. Watt] is assisted in its management by Professor D. Weatherall
and Dr. J. Hatzfeld and by an advisory board of 9 European Scientists
whose names are listed in Table 1.
Collaborative projects cover 4 areas for which the participants
possess considerable expertise. These are:
(i) Procedures for identifying the haemopoietic stem or very primitive
progenitor cells;
(ii) assays for human haemopoietic stem cells (such as in vitro
culture assays and animal models)
and ex vivo expansion of stem cells and their progeny;
(iii) the regulation of stem cells (e.g. by cytokines and extracellular
matrix molecules) and the regulation of genes expressed by stem
cells (e.g. cell surface adhesion and cytokine receptors; cell cycle
components; signal transduction molecules and transcription factors)
; (iv) clinical uses for stem cells (e.g. gene therapy for curing
genetic inherited diseases and gene and cell or gene therapy for
treating neoplastic diseases such as the leukaemias) .

The concerted action, because of its size, is now divided into 4
subgroups, to be co-ordinated as follows:
Subgroup 1 Cytokine subgroup Drs. J. Hatzfeld and G. Migliaccio
Subgroup 2 Gene Transfer and Cell Therapy: Professors A.A. Fauser,
A.M.Gianni, P. Mannoni, and w. Ostertag
Subgroup 3 Cell Markers: Professor w. Knapp, D. Birnbaum, R. Ploemacher
and J. visser
Subgroup 4 Cord Blood Banks Professor E. Gluckmann, J. Rows and
N. Testa
Subgroup 5: Animal Models: Dr B. Peault
Most of the papers being presented in the European Concerted Action
Session of this Advanced Workshop relate to gene transfer and therapy.
For these studies, we need to know:
(a) which tissue provides the best and most appropriate source of
haemopoietic progenitor cells for the uses specified above;
(b) whether genes must be transferred into the haemopoietic stem
cell or into their more mature progeny;
(c) how to identify the stem cell and its progeny and whether there
is a need to purify these cells for gene transfer studies;
(d) how to target the appropriate gene into the appropriate cell;
(e) how the introduced genes are regulated;
(f) if the introduction of genes results in the disruption of normal
genetic control mechanisms or induces an immune response.
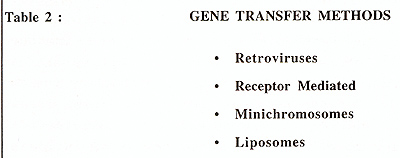
There are several methods currently available for gene transfer
and these are listed in Table 2, and these have recently been reviewed
by Moritz & Williams, (1994) .Both retroviral and receptor mediated
gene transfer methods require interaction with molecules on the
surface of the cells to be targeted. Within this concerted action,
the molecules being studied include cell surface adhesion molecules,
receptors involved in cell-cell, cell-matrix and cell-viral interactions,
and cytokine receptors
(such as novel tyrosine kinase receptors) .Molecules, within these
families, that are of particular interest to our laboratories are
CD34, Thyl, MDRI, ICAM-3, PECAM-l or CD3l, HLADR, CD33 and CD38
which allow identification and/or discrimination of primitive haemopoietic
progenitor cell subsets. This review will be divided into two parts.
The first section will describe a panning protocol for obtaining
highly enriched human haemopoietic precursors while maintaining
the phenotypically most primitive haemopoietic subsets, while the
second section will review three cell surface adhesion molecules,
PECAM-l (CD3l) , ICAM-3 (CDSO) and CD33, that may be expressed on
the most primitive haemopoietic stem cells.
Conclusions
A method for isolating CD34+ cells to a high degree of purity
has been described. Three adhesion molecules, PECAM-l, CD33 and
ICAM-3, are expressed in high amounts on all or on a proportion
of these CD34+ cells which appear to encompass the most primitive
haemopoietic progenitors. Although several studies have shown that
these molecules are functional on mature cells at least in vitro,
very little information is available on the function of these molecules
on haemopoietic progenitors, on where splice variants are expressed
during haemopoietic commitment and if the molecules are altered
by leukaemic transformation. These questions need to be addressed.
In addition, regulatory elements of these genes may provide a useful
means for regulating the expression of introduced genes into different
subsets of cells within the haemopoietic lineage.
| 
