|
* Department of Cell Biology, The Weizmann Institute of Science, Rehovot 76100, Israel
The generation of metastases by neoplastic cells constitutes the maill problem ill tumor malignancy. Metastasis is a multistep process in which each of the sequential steps IS controlled by different properties of the d.isseminating tumor cells. The host's capacity to recognize metastatic, as against nonmetastatic, cells of the tumor cell population could exert a controlling effect on each of the stages culminating in the progressive growth of metastases. Recognition of cel.lsurface antigenic epitopes on metastatIc cells via T lymphocytes would be restricted by cell-surface class I glycoproteins coded by the major histocompatibility complex (MHC). In mice, such glycoprotems are coded by the H -2D and H -2K. genes of the MHC, and differences in their expression on metastatic, as distinct from nonmetastatic, cells of a given tumor could therefore elicit different T cell effector responses, which would then determine the fate of the disseminating tumor cells. The feasibility of an MHC control of the metastatic process attracted us during investigations of the unique properties of a metastatic carcinoma originated in a C57BL (H -2b) mouse, the 3LL Lewis lung carcinoma. Following transplantation in syngeneic animals, the 3LL carcinoma generates spontaneous lung metastases while growing locally at any site of transplantation. However this tumor differs from all normal tissues and from many other tumors in its capacity to grow in allogeneic recipients. Yet, metastases were generated only when the tumor grew in syngeneic animals [ I ]. The allograft response elicited by the local tumor could not arrest the local growth, but was sufficiently powerful to prevent the growth of metastatic lung nodules. Subsequent experiments indicated that the spontaneous lung metastases behaved as "secondary" grafts, being rejected by .the alloreactive T cells that had been elicited by the local graft [2]. When the 3LL cells were injected intravenously to simIlar allogeneic mice, lung tumors developed as "primary" grafts and these did grow. progressively [2]. It thus appeared that In an allogeneic recipient the local tumor can resist an immune response, which prevents the growth of its spontaneous lung metastases. This raised the question as to whether an immune response elicited by the growing local tumor in syngeneic reci.pients could similarly prevent the progression of spontaneous metastases in syngeneic animals and whether the probability of forming metastases by individual tumor cells grown in syngeneic mice is 0 a function Of their immunogenic properties, which ill turn might be a function of the expression of the restricting class I MHC antigens on the neoplastic cells.
Table 1. Metastases and H-2 expression
of 3LL subclones 
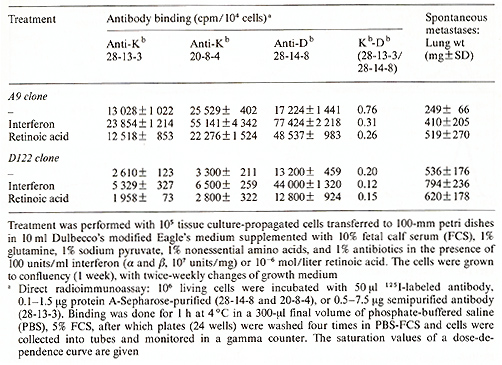
Our approach to the question as to whether differences in the expression of H-2Db versus H-2Kb glycoproteins (the class I antigens of the mouse MHC) control the metastatic potency of 3LL cells was triggered by earlier experiments in our laboratory. In these we aimed at determining the minimum genetic identities between the tumor strain of origin and the host's phenotype that are required for the generation of metastases. We found that identities at the H-2Db gene and the non-MHC background are sufficient for metastasis formation, whereas the H-2K phenotype of the recipient was completely irrelevant [ I]. It then turned out that identity at the H-2Kb was unnecessary, because the 3LL tumor hardly expressed the H-2Kb on its cell surface [3]. The question which thus arose was whether the absence of H-2Kb expression concomitant with the presence of H -2 Db determined the metastatic potency of the tumor cell population. To answer this question we cloned 3LL cells in soft agar and tested the metastatic potency of individual clones. We found that the clones differ in their capacity to generate spontaneous lung metastases when grown intrafootpad (i.f.p.) in syngeneic animals. As previously demonstrated for other tumors [4], this tumor cell population varied in the metastatic potency of its individual cells. To test whether there is a correlation between the metastatic properties of individual clones and the expression of MHC genes, we used monoclonal antibodies 28-13-3 and 20-8-4, which identify H-2Kb molecules, and antibody 28-14-8, which identifies H-20b molecules [5]. We analyzed 30 clones by direct radioimm unoassay and with the fluorescence-activated cell sorter. We found (Table I) that the lower the H-2Kb/H-2Db ratio, the higher was the metastatic potential of the cloned cells [6]. Table 2. The effect of interferon and
retinoic acid on MHC cell surface expression and metastasis
To examine whether the relative expression of class I antigens
of the MHC was causally related to its metastatic phenotype, we
attempted to alter the H-2Kb /H-2Db ratio, and then to test whether
such alteration will change the metastatic potency of the cells.
For this purpose we treated in vitrocloned tumor cells with either
interferon alfa+ß (a stimulator ofH-2 synthesis) or with retinoic
acid. Cells of two clones were used in these experiments: The low
metastatic A9 clone that expresses both the H-2K and the H-2D glycoproteins,
and the highmetastatic D 122 clone that expresses the H-2Db molecules
but lacks H-2Kb expression. It was found that interferon caused
an increase in both K and 0 expression of both A9 and D 122 cells,
yet the net increase in H-2Db expression was significantly higher
than that of H-2Kb expression, thus lowering the H-2K/H-2D ratio
(Table 2). These changes were associated with a significant increase
in the metastatic load produced by both the A9 clone and the DI22
clone. Treatment with retinoic acid did not affect H-2K expression,
but increased significantly H-2Db production, lowering the H-2K/H-2D
ratio even further. This again increased the metastatic load produced
by DI22 cells, and converted the low metastatic A9 clone to a high
metastatic phenotype (Table 2). Thus far we have not found a chemical
signal which would cause an increase in the H-2K/H-2D ratio and
thereby effect a decrease in the metastatic potency of the cells.
Very recent experiments with epsilon-interferon have indicated that
this interferon is more effective than interferon a+ ß in induction
of the cell surface expression of H-2Db and, especially, of H-2K
b molecules on A9 and D 122 tumor cells (Fig. I). While testing
for spontaneous lung metastases we observed no changes in the low
metastatic phenotype of A9 clone and a reduction in the number of
metastatic nodules formed by the high metastatic Dl22 clone. 
We then considered whether the low H-2K/H-2D ratio determines a metastatic phenotype because it confers a low immunogenic potency on the neoplastic cells. I. Growth and Metastasis in Allogeneic Recipients
Clone A9 behaves as a regular incompatible immunogenic allograft in allogeneic mice. To test whether the class I antigens alone on the A9 clone could elicit rejection of the grafted tumor, 105 A9 or Dl22 cells were inoculated to groups of H-2-recombinant mice on a C57BL/ 10 background [7]. We used BIO.HTG (KdDb), B10.D2 (KdDd), and BI0.A(4R) (KkDb) mice. Clone A9 (KbDb) grew in 91 10 BIO.HTG mice at a slower rate than in C57BL/6J mice. Only partial and slow growth was observed in B 10.D2 mice (51 II ), and the A9 clone grew in only one of nine mice of the BI0.A(4R) strain. In contrast, Dl22 grew in C57BL/6J and in the three recombinant strains at a similar rate. Testing for metastases, we found that Dl22 metastasized in C57BL/6J (KbDb), BIO.HTG (KdDb), and BI0.A(4R) (KkDb), but not in B10.D2 (KdDd) mice. The Kbpositive A9 cells grew partially in recombinant mice, while Dl22 grew progressively and metastases were rejected only in B10.D2 (Dd) mice. Thus, the higher immunogenic effect of clone A9, compared with the D 122 clone, is a function of an im mune response elicited by the H-2K deter minant.
Following the in vivo observations, we tested the cytotoxic T lymphocyte (CTL) responses evoked by cells of A9 and Dl22 clones in syngeneic hosts. C57BL/6J mice received 5 X 10 high 4 or 10 high 5 A9 or Dl22 tumor cells by intradermal injection. At 12 days after the immunization, spleen cells were removed and stimulated in vitro for 5 days on mono layers of irradiated and mitomycin C-treated A9 or Dl22 cells. The cytotoxic activity of these spleen cells was assayed against A9 and D 122 target cells in a 16- h indium-lll release assay. Figure 2 demonstrates that A9 induced high levels of cytotoxic activity, which was manifested against A9 cells and to a lesser exten t against D 122 target cells. D 122 cells induced a lymphocyte population that manifested low cytotoxic activity against Dl22 or A9 target cells. Thus, the in vitro interaction of immune lymphocytes with nonmetastatic A9 cells led to the destruction of the tumor cells, whereas lymphocytes interacting with D 122 cells were significantly less efficien t in destroying the tumor cells.

Low metastatic clones such as A9 were shown to bind anti-H-2Kb
and anti-H-2Db antibodies, while high metastatic clones such as
D 122 bound only H-2Db antibodies (Table 2). We tested the molecular
similarity of the MHC glycoproteins to H-2bmolecules of C57BL/6J
spleens. Immunoprecipitation of 125I cell surface-labeled and 35S-methionine-labeled
extracts of A9 and DI22 clones showed a strict correlation between
synthesis and cell surface expression. In D122 and in the parental
3LL cells, but not in A9, synthesis of K b molecules was suppressed
[6]. The 45K proteins precipitated by anti-H-2b serum or monoclonal
anti-H-2Kb (from clone A9) were similar in migration to molecules
precipitated from C57BL/6J splenocytes, and a l2K ß2-microglobulin
molecule was coprecipitated. Separation on lentil-lectin Sepharose
showed that most of the H-2bencoded proteins were in their glycosylated
form [6]. To obtain a better understanding of the transcriptional
level of the K gene suppression in metastatic clones, we used Northern
blot hybridization to analyze the mRNA from clones A9, D122, and
3LL as compared to liver mRNA and RNA extracted from metastatic
and nonmetastatic clones of another metastatic tumor, the TlO sarcoma
(TIO sarcoma is an F1 (H-2bX H-2k) tumor that expresses only D end
products of the MHC). We used four probes: (a) a genomic Kb, 5'
region probe, H8Pst8 [8]; (b) a cDNA, H -2d, 3' region probe, pH2IIa
[9]; (c) a cDNA, H-2, 5' region probe, pH2III [9]; and (d) a human
HLA-B9 cDNA probe. All these probes hybridize to both K and D end
transcripts. Figure 3 shows that the normal H-2 transcript of 2
kb that is expressed in the liver is also expressed at a high level
in clone A9 and in the 3LL line. A lower level of transcription
of the 2-kb mRNA is observed in clone D 122 and in the TIO sarcoma
clones, as both DI22 and all TIO clones lack expression of K end
products. Besides the normal 2-kb transcripts, an abnormal RNA of
5.5-6 kb was observed in all tumor clones [ 10]. This transcript
hybridized to the three 5' region probes but not to the 3' region
probe. The origin of this large RNA is not yet known, but it may
result from the insertion of a foreign DNA into the H -2K gene of
the tumors. In this event the large RNA could represent a transcript
contain ing H-2 sequences plus other sequences, and this could explain
the inactivation of the H-2K gene. Southern blot analysis, although
very complex, revealed new fragments hybridizing to the H-2 probes
when the genomic DNA from tumor clones was digested with EcoRI,
BamHI, Xbal, or SStI and compared with liver DNA of the same mouse

strains (C57BL for 3LL clones and Fl for TIO clones). Differences
were also observed between clones A9 and D 122. The possibility
that a mutation or insertion in H -2K genes might result in the
loss of expression of an H-2 molecule, giving rise to clones insensitive
to the host immune system, is investigated. 
The co-expression of the two class I antigens, which characterizes the low or nonmetastatic clones of 3LL, also characterizes most nucleated normal somatic cells, whereas both early embryonal cells and nondifferentiated teratocarcinoma cells lack expression of H-2 molecules. Do other gene products of the metastatic versus the nonmetastatic phenotypes signify differences in state of differentiation? Of particular interest from this viewpoint is the fos gene. Expression of c-fos, the cellular counterpart of the FBJ osteosarcoma viral onc gene, was shown to correlate with the induction of differentiation in human myeloid leukemia line WEHI-3B [11]. Transfection of F9 teratocarcinoma cells by a cloned fos gene was shown by Muller and Wagner to induce differentiation of teratocarcinoma cells to endoderm-like cells [ 12]. In a recent study of the organization and expression of onc genes in metastatic and nonmetastatic clones we observed that the c-fos proto-onc gene is expressed in the low metastatic clone A9 at high levels, while the high metastatic clone D122 does not contain c-fos-related m-RNA (Fig.4). A low level of cfos transcription was also observed in parental 3LL cells. Expansion of these results showed that other low metastatic 3LL clones also expressed c-fos. I n addition, we found that the c-myc oncogene was amplified 60-fold in all 3LL clones. Thus, the level of c-myc mRNA is very high in A9 and D122 clones, as well as in the parental 3LL line. Figure 4 demonstrates, using the same Northern blot, that while c-myc is expressed in all three cell types, c-fos is expressed mainly in the low metastatic A9 clone. Is the expression of c-fos gene product and a full expression of H-2K and H-2D gene products an indication of a more differentiated state of the A9 low metastatic clone than of the metastatic D 122 clone, and is such a differentiation step crucial in the control of metastatic spread by the host? We are currently investigating these questions.
This investigation was supported by PHS grant no. CA 28139 awarded by the National Cancer Institute, DHHS, USA.
I. Isakov N, Feldman M, Segal S (1981) Control of progression of local tumor and pulmonary metastases of the 3LL Lewis lung carcinoma by different histocompatibility requirements in mice. J N C I 66: 919 2. lsakov N, Feldman M, Segal S (1982) An immune response against the alloantigens of the 3LL Lewis lung carcinoma prevents the growth of lung metastases but not of local allografts. Invasion and Metastasis 2: 12 3. Isakov N, Katzav S, Feldman M, Segal S ( 1983) Lewis lung carcinoma cells possess immunogenic H-2D b region gene products but lack H-2Kb region products. J N C I 71:139 4. Fidler IJ, Kripke ML (1977) Metastasis re sults from pre-existing variant cells within a malignant tumor. Science 197:893 5. Ozato K, Sachs DH (1981) Monoclonal anti bodies to MHC antigens. Hybridoma antibodies reacting to antigens of H-2 b haplotype reveal genetic control of isotype expression. J Immunol126: 317 6. Eisenbach L, Segal S, Feldman M (1983) MHC imbalance and metastatic spread in Lewis lung carcinoma clones. Int J Cancer 32:113 7. Eisenbach L, Hollander N, Greenfeld L, Yakor H, Segal S, Feldman M (1984) The differential expression of H-2K versus H-2D antigens, distinguishing high-metastatic from low-metastatic clones, is correlated with the immunogenic properties of the tumor cells. Int J Cancer 34: 567 8. Weiss E, Golden L, Zakut R et al. (1983) The DNA sequence of the H-2Kb gene: Evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibility antigens. EMBO J 2:453 9. Steinmetz M, Erelinger JG, Fisher D et al. (1981) Three cDNA clones encoding mouse transplantation antigens: Homology to immunoglobulin genes. Ce1124: 125 10. Eisenbach L, Hollander N, Segal S, Feldman M (1985) The differential expression of class I major histocompatibility complex antigens controls the metastatic properties of tumor cells. Transplan tation Proc 17: 729 II. Gonda TJ, Metcalf D (1984) Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature 310:249 12. Müller R, Wagner GF (1984) Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature 311: 438 |