|
01* With minor variations this paper has been presented
at three symposia during the summer of 1980 : A. Abstract The oncogenic properties of sarcoma, acute leukemia, and lymphatic leukemia viruses are interpreted in terms of their genetic structures. Highly oncogenic sarcoma and acute leukemia viruses are shown to contain transforming onc genes which are different from the three virion genes (gag, pol, and env) essential for replication. Biochemical and genetic approaches to define onc genes are discussed. The hallmark of retroviral onc genes is shown to be a specific RNA sequence that is unrelated to essential virion genes. On this basis five different classes of onc genes can be distinguished in the avian tumor virus group alone: two of these, the onc genes of Rous sarcoma virus (RSY) and avian myeloblastosis virus (AMY), share one design. Their coding sequence is a specific RNA section which either replaces env [RSY( -), AMY] or maps adjacent to the 3' end of env (RSY). Expression of this class of onc genes is mediated via subgenomic mRNAs containing sequences from the 5' end of viral RN A spliced onto the onc gene coding sequences. The onc gene product of RSY has been identified as a 60,000-dalton phosphoprotein. Three other classes of onc genes, namely, those of the myelocytomatosis (MC29) subgroup of viruses, avian erythroblastosis virus (A£V), and Fujinami sarcoma virus (FSY), share another design. Their coding sequences are hybrids consisting of specific as well as of gag or gag and pol gene-related elements. The products of these onc genes, translated from full size genomic RNA, are hybrid proteins carrying gag or gag and pol determinants in addition to specific sequences. They are phosphorylated and range in size from 75,000 to 200,000 daltons. Since viruses with totally different onc genes can cause the same disease (namely, RSY, FSY, A£Y, and MC29 cause sarcoma and A£V, AMV, or £26 and MC29 cause erythroblastosis), it is concluded that multiple mechanisms involving multiple cellular targets exist for sarcomagenic and leukemic transformation of the avian cell. Comparisons between viral onc genes of the RSY -design and in particular those of the hybrid design and onc-related chromosomal DNA sequences of the cell suggest qualitative differences. Hence viral onc genes are not simply transduced cellular genes, and cellular sequences related to viral onc genes appear not directly relevant to cancer. It follows that viral onc genes are unique and more than the sum of their parts related to cellular DNA and to replicative genes of retroviruses. We speculate that onc genes also may plan a role indirectly in cancers caused by lymphatic leukemia viruses, although these viruses are not known to contain such genes. Table 1. Oncogenic properties of
retroviruses 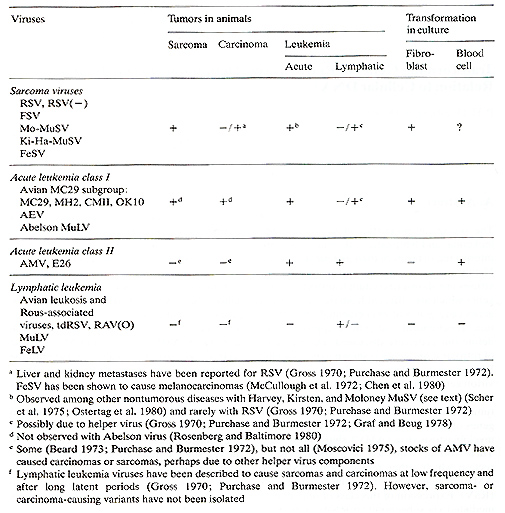
Retroviruses cause sarcomas, carcinomas, acute and lymphatic leukemias, or no disease in animals ( Gross 1970; Beard et al. 1973 ; Tooze 1973; Levy 1978; J arrett 1978; Duesberg 1980; Essex 1980). Table 1 shows schematically the pathology of representative re troviruses of the avian, murine, and feline tumor virus groups. The avian, murine, and feline sarcoma viruses predominantly cause sarcomas and transform fibroblasts in culture. The Harvey (Ha), Kirsten (Ki), and Moloney (Mo) murine sarcoma viruses (MuSV) and rarely avian Rous sarcoma virus (RSV) also cause erythroid leukemia ( Gross 1970; Scher et al. 1975; Ostertag et al. 1980; Duesberg 1980). This has not been observed with avian Fujinami virus (FSV) (Lee et al. 1980). Feline sarcoma virus (FeSV) in addition to sarcomas also causes melanocarcinomas (McCullough et al. 1972; Chen et al. 1981). The avian acute leukemia viruses of the MC29 subgroup and erythroblastosis virus (AEV) that transform fibroblasts [therefore termed "class I" (Duesberg 1980)] and hematopoietic cells in culture have broad oncogenic spectra including sarcomas and carcinomas in addition to acute leukemias in the animal (Beard et al. 1973 ; Graf and Beug 1978). However, the fibroblast-transforming murine Abelson leukemia virus has not been reported to cause sarcomas and carcinomas (Rosenberg and Baltimore 1980). By contrast the avian acute leukemia viruses AMV and E26 that do not transform fibroblasts in culture [ therefore termed "class II" (Duesberg 1980)] have rather specific oncogenic spectra in the animal, where they cause myeloid and erythroid leukemias (Beard et al. 1973 Moscovici 1975 ; Graf and Beug 1978). The viruses listed thus far have in common that they transform quickly, within 1-2 weeks, and that transformation is an inevitable consequence of infection in susceptible animals. This implies that transforming onc genes are integral parts of the genomes of these viruses. This appears not to be true for the majority of naturally occurring retroviruses, the lymphatic leukemia viruses. These are rather ubiquitous, nondefective viruses that often cause viremias but rarely and in particular not simultaneously cause leukemias ( Gross 1970 ; Tooze 1973), as for example in chickens (Rubin et al. 1962 ; Weyl and Dougherty 1977), mice (Gardner et al. 1976 ; Levy 1978 ; Cloyd et al. 1980), or cats (Jarrett 1978 ; Essex 1980). The transformation-defective (td), srcdeletion mutants of RSV have the same biologic (Biggs et al. 1972) and genetic properties (Wang et al. 1976) as the lymphatic leukemia viruses (Fig. 1). The RNA genome of these viruses contains a 3'terminal c-region and all three essential virion genes in the following 5' to 3' order: gag ( for internal virion proteins or group-specific antigens), pol (for RNA dependent DNA polymerase), and env (for envelope glycoprotein). The c-region has regulatory functions in the reverse transcription of viral RNA and in the transcription of proviral DNA (Fig. 1) (Wang et al. 1975; Wang 1978; Tsichlis and Coffin 1980). The endogenous, nondefective ( containing all three virion genes) retroviruses of chicken, such as RAV(O) (Tooze 1973), and of mice, such as xenotropic viruses (Levy 1978 ; Cloyd et al. 1980), are inherited according to Mendelian genetics. These viruses probably never cause a disease directly and appear to differ from the more pathogenic lymphatic leukemia viruses in minor genetic elements, including the c-region that influences virus expression (Tsichlis and Coffin 1980 ; Lung et al. 1980 ; Cloyd et al. 1980). Since transformation is not or is only rarely a consequence of replication by any of these viruses and only occurs after considerable latent periods, these viruses may not contain authentic onc genes. In the following we will describe the definition of onc genes of the rapidly transforming sarcoma and acute leukemia viruses. On this basis we will then ask whether the oncogenic specificity of some and the lack of specificity by other viruses is due to distinct onc genes or whether one onc gene can cause multiple forms of cancer. In addition, we review the question of the relationship between viral onc genes and cellular DNA. Finally, the question is addressed of how lymphatic leukemia viruses, which lack known onc genes, may cause cancer. The focus will be on avian tumor viruses, because their genetic structures are better defined than those of other viruses. This review extends two previous ones published recently (Duesberg, 1980; Bister and Duesberg 1980).
The only onc gene of retroviruses for which nearly complete genetic
and biochemical definitions are available is the srcgene of RSV.
In 1970 the src gene was formally distinguished from the three essential
virion genes of nondefective RSV by the isolation of a mutant that
was temperature-sensitive only in transformation but not in virus
replication (Martin 1970) and by the isolation of nonconditional
src-deletion mutants (Duesberg and Vogt 1970 ; Martin and Duesberg
1972). These transformation-defective src deletion mutants retain
all virion genes and are physically and serologically like wild
type RSV (Fig. 1) (Wang et al. 1975 ; Wang 1978). However the RNA
of the wild type measures 10 kb (kilobases) whereas that of the
tdRSV measures only 8.5 kb (Fig. 1) (Duesberg and Vogt 1970, 1973
; Lai et al. 1973 ; Beemon et al. 1974 ; Wang 1978). On the basis
of this difference src gene-specific RN A sequences were first defined
by subtracting from the 10-kb RNA of RSV with the genetic structure
5' gag-pol-env-src-c 3' the 8.5-kb RNA of the isogenic src deletion
mutant tdRSV with the genetic structure 5' gag-pol-env-c 3' (Fig.
1) (Lai et al. 1973). The 1.5 kb that set apart the wild type RSV
from the src deletion mutant were shown to be a contiguous sequence
that mapped near the 3' end of viral RNA (Wang et al. 1975). The
src gene was independently defined by recombination analysis in
which a src-deletion mutant with variant virion genes (5' gag"-pol"env"-c
3') was allowed to recombine with a nondefective RSV. All sarcomagenic
recom binants with variant virion genes had the genetic structure
5' gag"- pol"-env"-src-c 3 1 ) and hence had inherited the src gene
(Beemon et al. 1974 ; Wang et al. 1976; Wang 1978) (see also Fig.
1 ). It followed that the 1.5-kb sequence of RSV -specific RNA was
necessary for transformation. A major step towards proving that
the 1.5-kb src-specific RNA sequence was also (almost) sufficient
for transformation was ta- 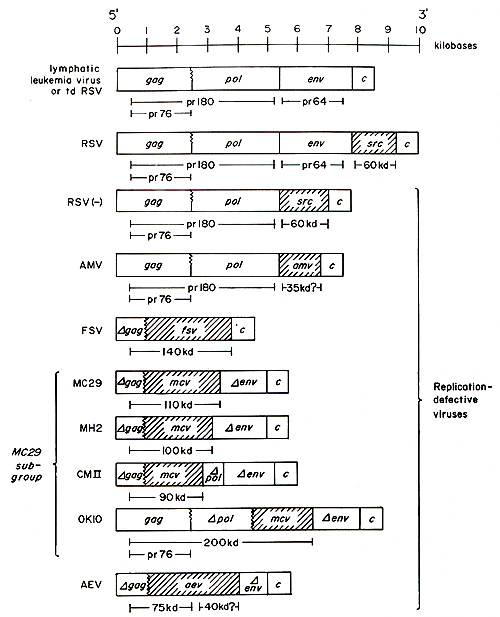
ken when a 60-kilodalton (kd) protein product was identified in RSV-transformed cells (Brugge and Erikson 1977) that was serologically unrelated to the gag, pol, and env virion proteins. Since the genetic complexity of the 1.5-kb RSV -specific RNA sequence and of the 60-kd src protein are about the same, the 1.5-kb RNA must encode most or all of the protein (Fig. 1). The same src-specific sequence has also been identified in a env-deletion mutant of RSV, termed RSV(- ) (Table 1, Fig. 1) (Wang et al. 1976), and recently also in a gag-, pol-, and env-defective sarcomagenic deletion mutant of RSV (Martin et al. 1980). Transformation by the gag-, pol-, and env-defective RSV is definitive proof that the src gene (but not the src-specific RNA sequence by itself) is sufficient for transformation. Expression of the src gene of RSV involves a mRNA which also includes sequences derived from the 5 I and 3 I ends of virion RNA that are shared with src-deletion mutants of RSV (Mellon and Duesberg 1977). This implies that the src-specific sequence of RSV defined by deletion and recombination analysis does not act independently and may by itself not be sufficient for transformation. Since the definition of the src gene of RSV most other acutely transforming retroviruses have been shown to contain specific sequences unrelated to essential virion genes. Such sequences appear to be the hallmark of highly oncogenic viruses, and they represent most or at least part of their oncgenes (see below). To date the oncogenic retroviruses are the only class of viruses which have onc genes that are nonessential for virus replication. The only known function of these genes is their oncogenicity. D. Identification of the Genome and Definition of the onc Genes of Replication- Defective Oncogenic Viruses The definition of the onc genes of highly oncogenic viruses other than RSV is less advanced than that of src. This is because all highly oncogenic retroviruses, with the exception of RSV, lack essential virion genes and consequently are replication defective. The genetic phenotype of most defective sarcoma and acute leukemia viruses is: gag-, pol-, env-, onc+ (Fig. 1) (Tooze 1973; Bister and Vogt 1978; Graf and Beug 1978). Due to this phenotype classical deletion and recombination analysis cannot be used to define onc genes as was the case with src. An oncdeletion of such a virus (i.e., gag-, pol-, env-, onc-) would obviously be undetectable by classical techniques measuring viral gene expression. Likewise the lack of secondary markers would complicate or prevent recombination analysis of onc genes of defective viruses.
III. Genetic Evidence That Specific and gag-Related RNA Sequences
of Avian Class I Acute Leukemia Genetic evidence was used to determine whether the specific sequences of defective transforming viruses are necessary for transformation. Since due to the absence of selective markers other than onc, suitable recombinants have not as yet been prepared in the laboratory, different isolates of closely and distantly related avian acute leukemia viruses were used as substitutes of recombinants. The RNAs of these viral isolates were compared to each other by electrophoretic size analysis, by hybridization with cDNAs of various avian tumor viruses, and by mapping RNase T 1-resistant oligonucleotides (Duesberg et al. 1977; Bister et al. 1979; Roussel et al. 1979; Duesberg et al. 1979; Bister et al. 1980a; Bister and Duesberg 1979, 1980). Such comparisons show that four different isolates of avian acute leukemia viruses, whose oncogenic spectra are closely related (Table 1 ) (Beard et al. 1973; Graf and Beug 1978), namely, MC29, MH2, CMII, and OK10, also have closely related genetic structures (Fig. 1). The hallmark of each viral RNA is an internal, helper virus unrelated sequence of about 1.5 kb (Duesberg et al. 1977, 1979; Bister et al. 1979; Bister and Duesberg 1980; Bister et al. 1980a; Roussel et al. 1979). Because the specific sequences of the four viruses are closely related and because the sequence was first identified in MC29 virus, it has been termed mcv. The mcv sequence is the structural basis for the classification of the four viruses into the MC29 subgroup of avian RNA tumor viruses (Bister et al. 1979; Bister and Duesberg 1980; Bister et al. 1980a; Duesberg 1980). The size and the oligonucleotide composition of the mcv sequences appears to vary between approximately 1.5 and 2 kb in different viral strains (Fig. 1) (Duesberg et al. 1979; Bister et al. 1979; Bister et al., 1980 a). Based on oligonucleotide complexity the largest mcv sequence appears to be that of MC29 virus and the smallest one either that of CMII or of MH2 (Bister et al. 1980a). In MC29, CMII and MH2 the mcv sequence ist flanked at the 5' end by a partial ( Delta ) gag gene, termed Delta gag, and in OK10 by a complete gag followed bya Delta pol (Fig. 1). It is not clear as yet whether the Delta gag sequences ofMC29, MH2, and CMII have the same complexities. At the 3' end the mcv sequence of MC29 and OK10 is flanked by a Delta env gene, and that of CMII, by Delta pol (Fig. 1) [ env sequences are present in MH2 RNA, but the RNA has not been analyzed sufficiently to determine whether its mcv sequence borders at Delta env (Fig. 1 ) (Duesberg and Vogt 1979)]. These comparisons of the four MC29-subgroup viral RNAs show that: (1) The 5' parts of their Delta gag or gag sequences and most, but not all, of their mcv sequences are highly conserved; (2) their env-related sequences are related by hybridization but variable if compared at the level of shared and specific T 1-oligonucleotides (Bister et al. 1979; Duesberg et al. 1979; Bister et al., 1980a), and (3) they may have optional sequences such as the 3' half of gag and the Delta pol at the 5' end of mcv in OK 10 or the Delta pol at the 3' end of mcv in CMII (Fig. 1). There are probably optional parts of the mcv sequence itself, because its size appears to vary in different viral strains. It would follow that most of mcv is an essential specific correlate and Delta gag an essential, nonspecific (because it is shared with helper virus and other defective viruses; Fig. 1) correlate of viraloncogenicity. These genetic analyses are confirmed and extended if one includes AEV (Bister and Duesberg 1979) and FSV (Lee et al. 1980). Each of these viruses has a genetic structure similar to the viruses of the MC29 subgroup, with a Delta gag sequence at the 5' end and internal specific sequences, termed aev and fsv (Bister et al. 1980a), which are unrelated to essential virion genes, to src, to mcv, and to each other (Fig. 1). Thus, these viruses form an analogous series of defective transforming viruses. It follows that the internal specific sequence of each of these viruses is necessary but probably not sufficient for transformation, since oncogenicity of each of these viruses also correlates with a highly conserved Delta gag. Hence the onc genes of these viruses appear to be genetic units consisting of gag-related and specific RNA sequences.
Each of the class I avian acute leukemia viruses as well as FSV code for gag-related nonstructural phosphoproteins ranging in size from 75 to 200 kd (Fig. 1) (Bister et al. 1977; Bister et al. 1979; Hayman et al. 1979a,b; Bister and Duesberg 1980; Lee et al. 1980; Ram say and Hayman 1980; Bister et al. 1980a) .That these proteins are coded for by gag-related as well as specific sequences of viral RNA was deduced from in vitro translation of viral RNAs of known genetic structure (Mellon et al. 1978 ; Lee et al. 1980) and from peptide analyses of these proteins (Hayman et al. 1979a,b; Kitchener and Hayman 1980). This directly supports the view that the specific sequences of these viruses are not independent genetic units (Fig. 1). They function together with at least the 5' part of gag ( or all of gag and part of pol in OKI0) as one genetic unit (Fig. 1) (Mellon et al. 1978; Bister and Duesberg 1980; Lee et al. 1980; Bister et al. 1980a). To indicate that translation crosses the border between gag-re lated or between gag- Delta pol-related and specific sequence elements, these borders were drawn as undulated lines in Fig. 1, also indicating that their exact location is uncertain. Given the very similar oncogenic spectra of these viruses and assuming transforming function of these proteins, we deduce that the size differences among the 90-,100-,11 0-, and 200-kd gag-related proteins of CMII, MH2, MC29, and OKI0 directly confirm the point made above on the basis of RNA analysis, i.e., that the Delta gag/gag-Delta pol-rncv units include optional elements (see Fig. 1). Moreover the structure of the genetic unit coding for the 200-kd protein of OKI0 which contains a complete gag and a mcv sequence which replaces only a part of pol (Fig. 1) is of particular interest regarding the role of gag-related sequences in these proteins. Based on analogy with pol gene expression by nondefective viruses which proceeds via a gag-pol precursor protein (Fig. 1), the OKI0 protein could also be processed into a product that contains only Delta pol and mcv. The fact that such a protein is not found in infected cells (Ramsay and Hayman 1980; our unpublished observa tions) suggest again that the gag-related portion is essential for the function of this protein. The optional nature of the pol sequence in OKI0, already evident from its lack in other MC29 subgroup proteins, is underscored by the fact that it includes the pol sequences that map at the 3' end of mcv in CMII which are not part of the CMII protein (Fig. 1) (Bister et al. 1979, 1980a). Because the genetic units of the MC29 subgroup viruses that read Delta gag-rncv or gag-Delta pol-rncv share conserved 5' gag elements and most of mcv but differ in optional, internal sequences, it has been proposed that these genes and their protein products have two essential domains, one consisting of the conserved gag-related, the other of the conserved mcv-related sequences (Bister et al. 1980a). Since the known proteins coded for by the class I acute leukemia viruses do not account for genetic information of the 3' half of the viral RNAs, it cannot be excluded that 3' terminal sequences are also necessary for transformation. Nevertheless the variability of the 3 I terminal sequences, both in terms of oligonucleotide composition and in relationship to env and pol genes (Fig. 1), as well as the lack of evidence for protein products synthesi zed in infected cells argue that these sequences may not be translated. It was hypothesized, therefore, that these sequences may not play a direct role in transformation (Duesberg et al. 1979; Bister et al.1980a ; Bister and Duesberg 1980). In contrast, all genetic information of FSY, which has a genetic structure that is similar to that of class I acute leukemia viruses (Fig. 1), can be accounted for in terms of one known viral protein. Moreover, if one assumes that transformation requires a viral protein and that the viral RNA is translated in only one reading frame, one may argue that in the case of FSY the Delta gag-fsv sequence is not only necessary but also sufficient for transformation, since the genetic complexities of the 4.5-kb FSY RNA and of the 140-kd protein encoded by Delta gag-fsv are about the same (Fig. 1).
The AMY and E26 are acute leukemia viruses which fail to transform fibroblasts and cause no sarcomas and possibly no carcinomas, signaling a unique class of onc genes (Beard et al. 1973; Moscovici 1975; Graf and Beug 1978). Until recently the analysis of AMY and related viruses has been slow, because infectious virus typically contains a large excess of nondefective helper virus. This has been changed by the discovery of defective AMY particles which are released by AMY-transformed nonproducer myeloblasts. Such particles contain a 7.5-kb viral RNA and are infectious if fused into susceptible cells together with helper virus (Duesberg et al. 1980). Consistent with the ability of AMY to produce defective virus particles, the RNA was found to contain a complete gag and pol gene, and non producer cells contain 76-kd gag and 180-kd gag-pol precursor proteins (Fig. 1). However there is no evidence for gag or gag and pol related non structural proteins in AMY- transformed cells (Duesberg et al. 1980). Between poland a unique 3 I terminal c-region AMY contains a specific amv sequence of about 1.5 kb that is unrelated to those of any other acutely transforming avian tumor virus except E26 (Fig. 1 ) (Duesberg et al. 1980). It appears that the genetic structure of AMY resembles closely that of RSY(-) (Fig. 1). From this genetic structure it may be expected that the amv sequence codes possibly for a specific protein unrelated to gag and pol genes by a mRNA similar to that coding for the src protein of RSY (Mellon and Duesberg 1977) .I t would be expected that this protein has a transforming function. Preliminary evidence indicates that a 35-kd protein is translated in vitro from AMY RNA (Fig. 1) (Lee and Duesberg, unpublished work).
The onc genes described here have -as far as defined -two different designs: those with a coding sequence that is specific and unrelated to essential virion genes, for example, the src gene of RSY and possibly the onc gene of AMY, and those with a coding sequence that is a hybrid of genetic elements derived from essential virion genes and specific sequences, for example, the onc genes of MC29, AEY, and FSY. The specific sequences of the hybrid onc genes are all inserted at their 5 'ends adjacent to partially deleted gag or pol genes (Fig. 1 ). By contrast the specific sequences of the onc genes, whose coding sequences lack genetic elements of essential virion genes, either replace env genes [RSY( -), AMY] or are inserted between env and the c-region (RSY) (Fig. 1). For convenient reference one design is referred to as "RSY design" and the other as "MC29 design" of onc genes according to the originally identified prototypes. The two onc gene designs also differ in their mechanism of gene expression: the hybrid onc genes, whose specific sequences replace gagor pol genetic elements, are probably translated from genomic viral RNA into gag or gag and pol related proteins like the pr76 gag or pr180 gag-pol proteins of nontransforming viruses that they replace (Fig. 1) .However, there is no evidence that the hybrid gene products are subsequently processed. By contrast the src gene of RSY and probably the amv sequence of AMY, which replace env or are inserted downstream of env, are translated from subgenomic mRNAs like the env genes of nontransforming viruses (Fig. 1) (Mellon and Duesberg 1977; Hayward 1977; Duesberg et al. 1980 ; Lee and Duesberg, unpublished work; Gonda and Bishop, personal communication). Hence the mechanism of gene expression of the two different onc designs closely follows that of the 5' most virion gene that they partially or completely replace (Fig. 1). To determine whether the gag-related ele ments of the hybrid onc genes are indeed essential for the probable transforming function of their proteins as our analyses suggest, it would be necessary to find transforming viruses in which the specific sequences of hybrid onc genes are not linked to gag or pol sequences. Conversely it would be interesting to know whether src or the amv sequence would have a transforming function if inserted into gag or pol genes. I t is thought that the 60- kd src gene product functions catalytically, probably as a phosphokinase (Erikson et al. 1980; Bishop et al. 1980), although there is evidence that kinase activity may not be the only function of the src gene product (Rübsamen et al. 1980; Bishop et al. 1980). By contrast the function of the gag-related proteins of avian acute leukemia and Fujinami sarcoma viruses, of the Abelson MuLV, and of the feline sarcoma viruses may not be solely catalytic. Although a kinase activity again appears as a candidate for a catalytic function of these proteins, this has not been demonstrated in each of these proteins. It appears associated with the gag-related proteins of some strains of Abelson virus (Witte et al. 1980) and, with some uncertainty, also with the proteins of the Gardner and Snyder- Theilen strains of feline sarcoma virus (Reynolds et al. 1980) but has not been found in some avian viruses with oncgenes of MC29 design (Bister et al. 1980b) and in the McDonough strain of feline sarcoma virus (Van de Ven et al. 1980). It is possible that these onc gene products have in addition to a possible catalytic function a structural function involving their gag-related elements. Analogous to the function of virion gag proteins, the gag portions of the nonstructural proteins of these viruses may function by binding to specific cellular and also to intracellular viral nucleic acid sequences. This specific binding may represent a regulatory function of a cellular catalytic activity or perhaps of a yet to be discovered catalytic activity of the gag-related proteins. This function would then correspond to one of the two domains diagnosed in the proteins of the MC29 subgroup viruses described above. The consistent difficulties in isolating temperature-sensitive onc mutants of these viruses that respond fast to temperature shifts (unpu blished experiments and personal communications) support the view that onc genes of the MC29 design may have a structural function.
Despite some insufficiencies in the definition of onc genes of defective viruses, it is clear that multiple, at least five different classes of specific sequences (src, mcv, aev,fsv, and amv) and hence probably five different onc genes exist in the avian tumor virus group alone. The number will increase if other viruses are analyzed and if viruses of other taxonomic groups are included. Some of these onc genes cause specific cancers in the animal: for example, RSV and FSV, which cause predominantly sarcomas, and AMV and E26, which cause specifically leukemias affecting myeloid or erythroid precursor cells or more primitive stem cells depending on the host [ chicken or quail (Moscovici and Löliger, personal communication)]. Other onc genes like those of MC29 and AEV may in addition to acute leukemias cause sarcomas and carcinomas (Table 1). The fact that different onc genes vary in specificity yet may cause the same cancer argues against a unique mechanism to transform a given class of differentiated cells. For example RSV, FSV, MC29, AEV, and even Kirsten MuSV (Galehouse and Duesberg 1976) all may cause sarcomas in birds and can also transform mammalian fibroblasts (not tested for FSV) (Quade 1979), although they contain totally different onc genes. Likewise, AEV, MC29, and E26 and AMV may cause erythroblastosis (Table 1) (Beard et al. 1973 ; Graf and Beug 1978), although their onc genes, except for those of AMV and E26 (Duesberg et al. 1980), are different. It is concluded that multiple mechanisms, involving multiple onc genes and onc gene products and presumably multiple cellular targets, exist for sarcomagenic and leukemogenic transformation. The fact that different onc genes cause the same cancers or that one onc gene may cause multiple cancers argues against the hypothesis that the transforming proteins of these viruses closely resemble specific cellular differentiation proteins and that transformation is a consequence of a competition between a specific viral transforming protein and a specific related cellular counterpart (Graf et al. 1980). The overlap among the oncogenic spectra of different onc genes suggests that different onc genes either interact with different specific cellular targets or that onc genes interact with nonspecific targets. A unique target for a given form of cancer would fail to explain why different onc genes may cause the same disease and why in some cases one onc gene may cause different cancers. The nature of cellular targets for viral transformation remains to be elucidated; it is believed to include factors determining susceptibility to virus infection and replication as well as intracellular substances that interact directly with viral transforming proteins. The helper virus unrelated specific sequences of acutely transforming avian, murine, and feline viruses have been shown to have closely related cellular counterparts (Scolnick et al. 1973,1975 ; Tsuchida et al. 1974; Frankel and Fischinger 1976; Stehelin et al. 1976b; Spector 1978a; Frankel et al. 1979; Sheiness and Bishop 1979; Hughes et al. 1979a,b; Souza et al. 1980; Oskarsson et al. 1980). This has lent support to the hypothesis that normal cells contain viral onc genes and that viral onc genes are transduced cellular genes (Huebner and Todaro 1969; Stephenson et al. 1979; Bishop et al. 1980). If correct, this hypothesis would predict paradoxically, that normal cells contain a number of viral or cellular onc genes that apparently are not subject to negative selection. (At present this number is around a dozen and is going up as more onc genes are defined. ) Thus normaly would be an admirable effort of cellular suppression of endogenous onc genes. Although their cellular relatives are even less well defined than most viral onc genes themselves, enough is known about them to deduce that viral onc genes are not in the cell but that some or most of their coding sequences have related counterparts in cellular DNA. 1. One example is srcof RSV : The only form in which the specific sequence of RSV has ever been shown to have transforming function is if it is part of the viral src gene. As such, this 392 sequence is expressed via a mRNA that shares 5 f leader and 3 f terminal c-region sequences with other virion genes (Mellon and Duesberg 1977). These virion sequences are not found in all veterbrates said to contain src-related sequences (Spector et al. 1978a) except in some strains of chicken. Moreover in chicken src-related and endogenous virion gene-related sequences are not located on the same restriction fragments of cellular DNA (Hughes 1979b) nor on the same chromosomes (Hughes 1979a). In addition the cellular src-related mRNA and DNA sequences appear not to be colinear with those of RSVs (Wang et al. 1977 ; Spector et al. 1978b; Hughes et al. 1979b). Hence concrete qualitative differences set apart the src of RSV and its relatives in normal vertebrate cells. 2. Another example of a close relationship between a viral onc gene and cellular alleles is the case of MuSV: Most of the helper virus unrelated 1.5-kb sequence of MuSV has a closely related, perhaps identical counterpart in the cell ( Oskarsson et al. 1980; Blair et al. 1980). However, molecularly cloned "MuSVspecific" DNA from the cell or from MuSV can only transform cultured mouse fibroblasts if it is first linked with (presumably noncoding) terminal sequences from MuSV or helper MuLV. Again concyete qualitative differences exist between the onc gene of MuSV and related DNA sequences of the cell. Moreover transformation by these modified MuSV-related sequences from the cell is abortive, and no infectious virus is recovered. Thus transformation by this kind of DNA and by infectious virus may prove not to be the same, although they appear indistinguishable based on the fibroblast assay. 3. The transforming genes of viruses which appear to be hybrids of structural and nonstructural viral genetic elements provide even more convincing evidence that viral onc genes and their cellular relatives are not the same thing: Although it has been shown that most but not all vertebrate cells contain sequences related to the helper virus unrelated part of MC29 and AEV, the helper virus-related elements of these viruses, in particular gag-related elements, do not have the same distribution and are not found in the same cells (Sheiness and Bishop 1979; Roussell et al. 1979). Hence gag-related sequences, thought to be an essential element of the onc genes of MC29 and AEV, are not part of the cellular sequences related to those genes. Moreover , the cellular sequences related to the 3-kb AEV -specific RNA sequence have recently been shown to be distributed over a 15-kb DNA segment that includes AEV -related and AEV -unrelated sequences (1. M. Bishop, personal communication). Likewise, the cellular DNA sequence, related to the Abelson murine leukemia virus-specific RNA sequence of 3 kb (Shields et al. 1979), has been shown to be distributed over a 12-kb DNA segment that must include Abelson virus unrelated sequences (Goff et al. 1980). Hence in these cases proviral DNA and related cellular DNA sequences are not colinear. It follows that the genetic units of class I acute leukemia viruses that consist of gag-related and specific RNA sequences (Fig. 1) have no known counterparts in normal cells. It is concluded that viral onc genes of the RSV design and in particular those of the MC29 design are different from related sequences present in the cell. The onc genes of the RSV design, like src and possibly amv (Fig. 1), may share most but possibly not all of their coding sequences with cellular homologs but differ from cellular relatives in essential regulatory elements. The onc genes of the MC29 design differ from cellular counterparts in coding (gag and po/-related sequences) as well as regulatory elements. Consequently, the cellular relatives of most viral onc genes are probably not present in the cell as functional onc genes as has been postulated (Huebner and Todaro 1969; Bishop et al. 1980) and hence are probably not directly relevant to transformation. Instead these cellular sequences may be relevant to the archaeology of viral onc genes. Viral onc genes probably have been generated by rare transductions of cellular sequences by nondefective viruses. To generate onc genes of the RSV design transduction must have involved illegitimate recombination with nondefective virus. In the case of Moloney MuSV specific deletions of the parental nondefective virus also had to occur (Dina et al. 1976 ; Donoghue et al. 1979; Chien et al. 1979; Blair et al. 1980). Until the cellular src-related sequence is characterized directly, it remains unclear whether in the case of RSV the coding sequence of src was transduced unchanged or after alteration when RSV was generated. Both transduction, involving again illegitimate recombination, as well as specific deletions of virion genes ( see Fig. 1) must have been necessary to generate the onc genes of the MC29 design from cellular and viral genetic elements. Such events are much less likely to occur than, for example, the transduction by phage lambda of a functional galactosidase gene. It is noted that experimental evidence compatible with the transduction of cellular sequences has led to the hypothesis that viral transformation is the product of enhancing the dosage of endogenous cellular onc genes by homologous equivalents from exogenous viruses (Bishop et al. 1980). We submit that sequence transduction is not synonymous with the transduction of unaltered gene function which would be a necessary corollary of the gene dosage hypothesis. It would appear that viral onc genes are unique and more than the sum of their parts related to cellular DNA and replicative genes of retroviruses.
Highly oncogenic viruses with onc genes such as those described here have only been isolated in relatively few cases from animal tumors (reviewed in Gross 1970; Tooze 1973; Duesberg 1980). By contrast leukosis and lymphatic leukemia viruses have been isolated from many viral cancers, in particular from leukemias (see above) (Gross 1970; Tooze 1973). Thus paradoxically the lymphatic leukemia viruses which lack known onc genes appear to be more relevant to viral carcinogenesis than retroviruses with known onc genes. However, it has been argued that onc genes also playa role in carcinogenesis caused by lymphatic leukemia viruses (Duesberg 1980). These onc genes may derive from endogenous, defective retrovirus-Iike RNAs known to exist in some normal cells (Duesberg and Scolnick 1977; Scolnick et al. 1979) or from cellular genes acquired by processes involving illegitimate recombination and specific deletions ( compare genetic structures shown in Fig. 1) . The necessity for such a secondary event to occur would explain the poor correlation between the distribution of lymphatic leukemia viruses and cancers in animals (see above ). The failure to find onc genes in most retroviral cancers may then reflect technical difficulties. These include the lack of suitable probes to detect viral RNA as the only characteristic viral structural component or nonstructural proteins as the only characteristic products of defective transforming viruses. Moreover, detection of a putative defective-transforming virus will be complicated by the fact that the ratio of defective-transforming to nondefective helper virus is low in typical stocks of defective helper virus complexes (Duesberg et al. 1977; Bister and Duesberg 1979; Duesberg et al. 1979; Lee et al. 1980). Thus, the analysis of viral onc genes may prove to be less academic than it appears at present -it may provide the tools and concepts necessary to understand all retroviral cancers.
We thank L. Evans, M. Nunn, and G. S. Martin for a critical review
of the manuscript and D. Baltimore, - Beard JW, Langlois AJ, Beard D (1973) Etiological strain specificities of the avian tumor virus. Bibl Haematol 39:31-44 - Beemon K, Duesberg PH, Vogt PK (1974) Evidence for crossing over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci USA 71 :4254-4258 - BiggsPM, Milne BS, Graf T, Bauer H (1972) Oncogenicityof nontransforming mutants of avian sarcoma virus. J Gen Virol 18: 399-403 - Bishop JM, Courtneidge SA, Levinson AD, Oppermann H, Quintrell N, Sheiness DK, Weiss SR, Varmus HE (1980) The origin and function of avian retrovirus transforming genes. Cold Spring Harbor Symp Quant Bioi 44:919-930 - Bister K, Duesberg PH (1979) Structure and specific sequences of avian erythroblastosis virus RNA: Evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci USA 76: 5023-5027 - Bister K, Duesberg PH (1980) Genetic structure of avian acute leukemia viruses. Cold Spring Harbor Symp Quant Bioi 44: 801-822 - Bister K, Vogt PK ( 1978 ) Genetic analysis of the defectiveness in strain MC29 avian leukosis virus. Virology 88:213-221 - Bister K, Hayman MJ, Vogt PK (1977) Defectiveness of avian myelocytomatosis virus MC29: Isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology 82: 431-448 -Bister K, Löliger H -C, Duesberg PH (1979) Oligoribonucleotide map and protein of CMII : Detection of conserved and nonconserved genetic elements in avian acute leukemia vIruses CMII, MC29 andMH2. J ViroI32:208-219 -Bister K, Ram say G, Hayman MJ, Duesberg PH (1980a) OK10, an avian acute leukemia virus of the MC29 subgroup with a unique genetic structure. Proc Natl Acad Sci USA 77:7142-7146 - Bister K, Lee W-H, Duesberg PH ( 1980b ) Phosphorylation of the nonstructural proteins encoded by three avian acute leukemia viruses and by avian Fujinami sarcoma virus. J Virol 36:617-621 - Blair DG, McClements WL, Oskarsson MK, Fischinger PJ, VandeWoude GF (1980) Biological activity of cloned Moloney sarcoma virus DNA: Terminally redundant sequences may enhance transformation efficiency. Proc Natl Acad Sci 77:3504-3508 - Brugge JS, Erikson RL (1977) Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature 269: 346-348 - Chen A, Essex M, Shadduck JA, Niederkorn JY, Albert D (1981) Retravirus encoded transformation-specific polyproteins: Expression coordinated with malignant phenotype in cells from different germ layers. PNAS, in press - Chien YS, Verma IM, Duesberg PH, Davidson N (1979) Heteroduplex analysis of the RNA of clone 3 Moloney murine sarcoma virus. J Virol 32: 1028-1032 - Cloyd MW, Hartley JW, KeneWP (1980) Iymphomagenicty of recombinant mink cell focus -inducing murine leukemia viruses J Exp Med 151: 542-552 - Dina D, Beemon K, Duesberg PH (1976) The 30S Moloney sarcoma virus RNA contains leukemia virus nucleotide sequences. Cell 9:299-309 -Donoghue BJ, Sharp PA, Weinberg RA (1979) Comparative study of different isolates of murine sarcoma virus. J Virol 32: 1015-1027 -Duesberg PH (1980) Transforming genes of retroviruses. Cold Spring Harbor Symp Quant Bioi 44:13-29 -Duesberg PH, Scolnick EM (1977) Murine leukemia viruses containing a rv30S RNA subunit of unknown biological activity, in addition to the 38S subunit of the viral genome. Virology 83:211-216 - Duesberg PH, Vogt PK (1970) Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci USA 67: 1673-1680 - Duesberg PH, Vogt PK (1973) RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology 54:207-219 - Duesberg PH, Vogt PK (1979) Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: Evidence for a second class of transforming genes. Proc Natl Acad Sci 76:1633-1637 - Duesberg PH, Bister K, Vogt PK (1977) The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci 74:4320-4324 - Duesberg PH, Bister K, Moscovici C (1979) Avian acute leukemia virus MC29 : Conserved and variable RNA sequences and recombination with helper virus. Virology 99:121-134 - Duesberg P, Bister K, Moscovici C (1980) Genetic structure of avian myeloblastosis virus released as defective virus particle from transformed myeloblasts. Proc Natl Acad Sci USA 77: 5120-5124 - Erikson RL, Collet MS, Erikson E, Purchio AF, Brugge JS (1980) Protein phosphorylation mediated by partially puri fied avian sarcoma virus transforming gene product. Cold Spring Harbor Symp Quant Biol 44: 907 -917 - Essex M (1980) Etiology and epidemiology of leukemia and lymphoma in outbred animal species. In: Yohn DS, Lapin BA, Blakesleee JR ( eds ) Advances in comparative leukemia research 1979. Elsevier/North-Holland, New York, pp 432-430 - Frankel AE, Fischinger PJ (1976) Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci 73:3705-3709 - Frankel AE, Gilbert PM, Porzig KJ, Scolnick EM, Aaronson SA (1979) Nature and distribution of feline sarcoma virus nucleotide se quences. J Virol 30:821-827 - Galehouse D, Duesberg PH (1976) RNA and proteins of Kirsten sarcoma xenotropic leukemia virus complex propagated in rat and chick cells. Virology 20:970-104 - Gardner MB, Henderson BE, Estes JD, Rongey RW, Casagrande J, Pike M, HuebnerRJ (1976) The epidemiology and virology of C-type virus associated hematological cancers and related diseases in wild mice. Cancer Res 36:574-581 - Goff SP, Gilboa E, Witte ON, Baltimore D (1980) Structure of the Abelson murine leukemia virus genome and the homologous ccllular genc: Studies with cloned virae DVA. Cell 22:777-785 - Graf T, Beug H (1978) Avian leukemia viruses. Interaction with their target cells in vivo and in vitro. Biochim Biophys Acta 516:269-299 - Graf T, Beug H, Hayman MJ (1980) Target cell specificity of defective avian leukemia viruses: Haematopoietic target cells for a given virus type can be infected but not transformed by strains of a different type. Proc Natl Acad Sci USA 77:389-393 - Gross L (1970) Oncogenic Viruses. Pergamon, New York Oxford London Paris -Hayman MJ, Royer-Pokora B, Graf T (1979a) Defectiveness of avian erythroblastosis virus: Synthesis of a 75k gag-related protein. Virology 92:31-45 - Hayman MJ, Kitchener G, Graf T ( 1979b ) Cells transformed by avian myelocytomatosis virus strain CMII contain a 90K gag-related protein. Virology 98: 191-199 - Hayward WS (1977) The size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol 24:47-64 -Huebner RJ, Todaro GJ (1969) Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci USA 64: 1087-1091 - Hughes SH, Payvar F, Spector D, Schimke RT, Robinson H, Payne GS, Bishop JM, Varmus HE (1979a) Heterogeneity of genetic loci in chickens: Analysis of endogenous viral and nonviral genes by cleavage of DNA with restriction endonuclease. Cell 18: 347-359 - Hughes SH, Stubblefield F, Payvar F, Engel JD, Dodgson JB, Spector D, Cordell B, Schimke RT, Varmus HE (1979b) Gene localization by chromosome fractionation: Globin genes are on at least two chromosomes and three estrogen-inducible genes are on three chromosomes. Proc Natl Acad Sci USA 76: 1348-1352 - Jarrett O (1978) Infectious leukemias in domestic animals. In: Neth R, Gallo RC, Hofschneider P-H, Mannweiler K (eds) Modern trends in human leukemia III. Springer, Berlin Heidelberg New York, pp 439-444 - Kitchener G, Hayman MJ (1980) Comparative tryptic peptide mapping studies suggest a role in cell transformation for gag-related proteins of avian erythroblastosis virus and avian myelocytomatosis virus strains CMII and MC29. Proc Natl Acad Sci USA 77: 1637-1641 - Lai MMC, Duesberg PH, Horst J, Vogt PK (1973) Avian tumor virus RNA. A comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci USA 20: 2266-2270 - Lee W-H, Bister K, Pawson A, Robins T, Moscovici C, Duesberg PH (1980) Fujinami sarcoma virus: An avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci USA 77:2018-2022 - Levy JH (1978) Xenotropic type C viruses. Curr Top Microbiol Immunol 79: 111-213 - Lung ML, Hering C, Hartley JW, Rowe WP, Hopkins N ( 1980 ) Analysis of the genomes of mink cell focus-inducing murine type C viruses: A progress report. Cold Spring Harbor Symp Quant Biol 44:1269-1274 - Maisel J, Klement V, Lai MMC, Ostertag W, Duesberg PH (1973) Ribonucleic acid components of murine sarcoma and leukemia viruses. Proc Natl Acad Sci USA 70:3536-3540 - Martin GS (1970) Rous sarcoma virus: A function required for the maintenance of the transformed state. Nature 227: 1021-1023 - Martin GS, Duesberg PH (1972) The a subunit in the RNA of transforming avian tumor viruses: I. Occurrence in different virus strains. II. Spontaneous loss resulting in non-transforming variants. Virology 47 : 494-497 - Martin GS, Lee WH, Duesberg PH (1980) Generation of non-defective Rous sarcoma virus by asymmetric recombination between deletion mutants. J Virol 36: 591-594 - McCullough B, Schaller J, Shadduck JH, Yolin DS (1972) Induction of malignant melanomas associated with fibrosarcomas in cats inoculated with Gardner-feline fibrosarcoma virus. J Natl Cancer Inst 48: 1893-1896 - Mellon P, Duesberg PH ( 1977) Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature 270:631-634 - Mellon P, Pawson A, Bister K, Martin GS, Duesberg PH (1978) Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci USA 75: 5874-5878 - Moscovici C (1975) Leukemic transformation with avian myeloblastosis virus: Present status. Curr Top Microbiol Immunol 71 : 79-101 - Oskarsson MK, McClements WL, Blair DS, Maizel JV, Vande Woude GS (1980) Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science 207: 1222-1224 - Ostertag W, Vehmeyer K, Fagg B, Pragnell IB, Paetz W, Le Bourse MC, Smadja-Joffe F, Klein B, Jasmin C, Eisen H (1980) Myeloproliferate virus, a cloned murine sarcoma virus with spleen focusforming properties in adult mice. J Virol 33: 573-582 - Quade K (1979) Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology 98: 461-465 - Ramsay G, Hayman MJ ( 1980) Analysis of cells transformed by defective leukemia virus OK 10 : Production of non-infectious particles and synthesis of pr76 gag and an additional 200,000 dalton protein. Virology 106:71-81 - Reynolds FW, Van de Ven WJM, Stephenson JR (1980) Feline sarcoma virus polyprotein P 115 binds a host phosphoprotein in transformed cells. Nature 286:409-412 - Rosenberg N, Baltimore D (1980) Abelson virus. In: Klein G (ed) Viral oncology. Raven, New York, pp 187-203 - Roussel M, Saule S, Lagrou C, Rommens C, Beug H, Graf T, Stehelin D (1979) Three new types of viral oncogenes of cellular origin specific for haematopoietic cell transformation. Nature 281 :452-455 - Rubin H, Fanshier C, Cornelius A, Hughes WF (1962) Tolerance and immunity in chickens after congenital and contact infection with avian leukosis virus. Virology 17: 143-156 - Rübsamen H, Ziemiecki A, Friis RR, Bauer H (1980) The expression of pp60 src and its associated protein kinase activity in cells infected with different transformation-defective temperature-sensitive mutants of Rous sarcoma virus. Virology 102 : 453-457 - Scher CD, Scolnick EM, Siegler R (1975) Induction of erythroid leukemia by Harvey and Kirsten sarcoma virus. Nature 256: 225-226 - Scolnick EM, Rands E, Williams D, Parks WP (1973) Studies on the nucleic acid sequences of Kirsten sarcoma virus: A model for the formation of a mammalian RNA-containing, sarcoma virus. J Virol 12:456-463 - Scolnick EM, Goldberg RJ, Siegler R (1975) A biochemical and genetic analysis of mammalian RNA-containing sarcoma viruses. Cold Spring Harbor Symp Quant BioI 39: 885-895 - Scolnick EM, Vass WC, Howk RS, Duesberg PH (1979) Defective retrovirus-Iike 30S RNA species of rat and mouse cells are infectious if packaged by Type C helper virus. J Virol 29: 964-972 - Sheiness D, Bishop JM (1979) DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol31 :514-521 - Shields A, Goff S, Paskind M, Otto G, Baltimore D (1979) Structure of the Abelson murine leukemia virus genome. Cell 18:955-962 -Souza LM, Strommer JN, Hillgard RL, Komaromy MC, Baluda MA (1980) Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci USA 77:5177-5181 - Spector DH, Varmus HE, Bishop JM (1978a) Nucleotide sequences related to the transforming genes of avian sarcoma virus are present in DNA of uninfected vertebrates. Proc Natl Acad Sci USA 75 :4102-4106 - Spector DH, Baker B, Varmus HE, Bishop JM (1978b) Characteristics of cellular RNA related to the transforming gene of avian sarcoma viruses. Cell 13:381-386 -Stehelin D, Guntaka R, Varmus HE, Bishop JM (1976a) Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol BioI 101 :349-365 - Stehelin D, VarmusHE, BishopJM, Vogt PK (1976b) DNA related to the transforming gene( s ) of avian sarcoma viruses is present in normal avian DNA. Nature 260: 170-173 - Stephenson JR, Khan AS, Van de Ven WJM, ReynoldsFHJr(1979) Type C retroviruses as vectors for cloning cellular genes with probable transforming function. J Natl Cancer Inst 63:1111-1119 - Tooze J (1973) The Molecular Biology of Tumour Viruses. Cold Spring Harbor haboratory, New York -Tsichlis PN, Coffin JM (1980) Role of the c-region in relative growth rates of endogenous and exogenous avian oncoviruses. Cold Spring Harbor Symp Quant BioI 44: 1123-1132 - Tsuchida N, Gilden RV, Hatanaka M (1974) Sarcoma virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci USA 71 :4503-4507 - Van de Ven WJM, Reynolds FH, Nalewaik RP, Stephenson JR (1980) Characterization of a 170,000 dalton polyprotein encoded by the McDonough strain of feline sarcoma virus. J Virol 35:165-175 - Wang LH (1978) The gene order of avian RNA tumor viruses derived from biochemical analyses of deletion mutants and viral recombinants. Annu Rev Microbiol 32:561-593 - Wang L-H, Duesberg PH, Beemon K, Vogt PK: Mapping RNase Tl-resistant oligonucleotides of avian tumor virus RNAs: Sarcoma specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol16: 1051-1070 - Wang L- H, Duesberg PH, Mellon P, Vogt PK ( 1976 ) Distribution of envelope-specific and sarcoma-specific nucleotide sequences from different parents in the RNAs of avian tumor virus recombinants. Proc Natl Acad Sci USA 73: 1073-1077 - Wang SY, Hayward WS, Hanafusa H (1977) Genetic variation in the RNA transcripts of endogenous virus genes in uninfected chicken cells. J Virol 24: 64- 73 - Weyl KS, Dougherty RM (1977) Contact transmission of leukosis VIrus. J Natl Cancer Inst 58: 1019-1025 - Witte ON, Dasgupta A, Baltimore D (1980) Abelson murine leukemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature 283: 826-831 |