Gregory R. Johnson 4, and Andrey S. Shaw 2,3 In: Zander AR et al. (eds) Gene Technolgy, Stem Cell and Leukemia Research, Nato ASI Series H: Cell Biology, Vol 94, Springer-Verlag, Berlin Heidelberg New York London, pp 321-331 |
|
1 Departments of Medicine and Cell Biology, 2 Pathology,
and
The receptor for erythropoietin is restricted to cells of mature erythroid and possibly megakaryocyte lineages. Studies in cell lines have suggested that cytokine receptors share a conserved signaling pathway for proliferation. We retrovirally transduced the erythropoietin receptor or a constitutively activated form of the EPOR into normal hematopoietic progenitors, including blast cell colonies. The EPO-R was able to support the proliferation and differentiation of early erythroid, early megakaryocytic, and macrophage progenitors, but not granulocyte progenitors. Blast cell colonies transduced with the EPO-R proliferate in response to EPO but the development of erythroid cells was not favored over other lineages. These results with normal cells suggest that some but not all cytokine receptors exhibit shared signaling pathways, and that EPO signaling alone is not sufficient to drive erythroid development. The Janus family of cytosolic tyrosine kinases mediate cytokine initiated mitogenic signals. We have determined that the EPO-R box 1 cytoplasmic motif is required for the binding and activation of JAK2. However, sequences outside the box 1 domain most likely regulate the specificity of JAK kinase association.
Erythropoietin (EPO) is a serum glycoprotein hormone required for the survival, proliferation and differentiation of committed erythroid progenitor cells, and is the principal hormone regulating the level of circulating red blood cells. The administration of recombinant human EPO to anemic patients suffering from chronic renal failure, AIDS, or bone marrow suppression due to chemotherapy has dramatically alleviated their need for blood transfusions. In contrast to many other hematopoietic growth factors, EPO acts primarily on relatively mature erythroid progenitors, and to a significantly lesser extent megakaryocyte progenitors, within the fetal liver and adult bone marrow (reviewed in (Krantz, 1991). The receptor for EPO is normally restricted in its expression to relatively mature cells of the erythroid and megakaryocyte lineage(Youssoufian, Longmore et al., 1993), and has also been reported to be expressed in the placenta(Sawyer, Krantz et al., 1989), by embryonic stem cells(Keller, Kennedy et al., 1993), on endothelial cells(Anagnostou, Lee et al., 1990), and on some neuronal-like celllines(Masuda, Nagao et al., 1993). The functional relevance of this developmentally diverse EPO-R gene expression is not clear, and quite apart from its function in hematopoiesis, EPO-Rs may play other roles in non-hematopoietic cells. In the case of the erythroid lineage, expression of the EPO-R is low or absent on immature progenitors such as BFU-E and is increased by the CFU-E stage of differentiation, before decreasing as the erythroid cells undergo terminal differentiation(Sawada, Krantz et al., 1990). Stimulation of adult BFU-E with EPO as a single stimulus fails to support the formation of erythroid colonies(Oai, Krantz et al., 1991). Whether EPO directly affects differentiation as well as proliferation is not clear. A murine EPO-R cONA, which stimulates EPO-dependent proliferation of several hematopoietic cell lines, was isolated by expression cloning and encodes a type I membrane-spanning protein of 66kO which is a member of the cytokine receptor superfamily(O'Andrea, Lodish et al., 1989). Studies have detected a single binding affinity for EPO (kO = 300-800pM) in heterologous hematopoietic cells, fibroblasts, or COS cells transfected with the EPO-R cONA. Binding studies on human and murine bone marrow and fetal erythroid progenitor cells have detected either one or two affinities for radioiodinated EPO (Youssoufian, Longmore et al., 1993). Two affinities for EPO may suggest the presence of other components which modulate the binding activity of the cloned EPO-R. However, it is unresolved whether there are indeed EPO-Rs of multiple affinities. A constitutively active (cytokine-independent) form of the EPO-R has been isolated from a retroviral transduction system and found to contain a single point mutation, resulting in an Arg to Cys change at residue 129 of the exoplasmic domain (Yoshimura, Longmore et al., 1990). The R129C receptors form disulfide-linked homodimers independent of hormone, yet retain the capacity to bind EPO with a single affinity (kD= 700pM)(Watowich, Yoshimura et al., 1992). Since several members of the cytokine receptor family are active as ligand-induced homo- or heteromultimers, the disulfide-linked dimers most likely mimics the structure of the hormone-bound form of the EPO-R and thus transmit a constitutive proliferative signal. Chimeric c-kit/EPO-R receptors transmit mitogenic signals in response to added c-kit ligand (Ohashi, Maruyama et al., 1994). When an EPO-R lacking the entire cytoplasmic region is coexpressed, in excess, with wild type EPO-R EPO-induced mitogenic signals are blocked ("dominant negative" receptor form) (Watowich, Hilton et al., 1994). Taken together these observations strongly suggest that the functional cell surface form of the EPO-R is a multimeric protein complex consisting of, at least, a homodimer of the cloned cDNA. Although the cytosolic domain of the EPO-R does not contain an obvious protein kinase domain, ligand induced multimerization of the EPO-R stimulates rapid and transient tyrosine phosphorylation of a number of cellular proteins, which are essential for EPO-induced proliferation (Miura, D'Andrea et al., 1991). Sequence analysis, and functional mutagenesis studies of members of the cytokine receptor superfamily of receptors have identified two small conserved motifs, box 1 and box 2, in the membrane proximal cytoplasmic domains, which are required for proliferative signals (Murakami, Narakaki et al., 1991). This membrane proximal domain has been shown to be necessary for binding and phosphorylation of Janus kinase family members (cytosolic, nonreceptor tyrosine kinases). There is some specificity for the JAK kinase member utilized by different receptors. For example, the EPO-R activates only JAK2, whereas the 11-6 receptor signal transducing component, gp130, activates either or both JAK1 and JAK2, depending upon the cell type expressing gp130 (Witthuhn, Quelle et al., 1993)(Stahl and Yancopoulos, 1993).
To identify features of the EPO-R that mediate its interaction with JAK2, we generated chimeric receptor proteins that contained the cytoplasmic domain of the EPO-R or gp130 fused to the extracellular and transmembrane domain of the VSV G protein. This modification allowed us to immunoprecipitate and immunoblot both EPO-R and gp130 receptors with the same monoclonal antibody and eliminated the variability of using different receptor antibodies for our comparison. Because the kinase activity of JAK2 is not detectable in the absence of cytokine stimulation we generated constitutively active forms of JAK1 and JAK2 by replacing the tyrosine kinase domain of the JAKs with an epitope tagged (myc) tyrosine kinase domain from p59fyn .Thus anti-myc monoclonal antibodies will immunoprecipitate the JAKs. The chimeric receptors and JAK kinases were transiently coexpressed in HeLa cells using the vaccinia-17 expression system to determine if cytokine receptors and JAK kinases could form stable complexes. The membrane proximal domain of the EPO-R was required for association with JAK2, and JAK1 did not associate with the EPO-R (Table 1). Specifically box 1, not box 2, was required for the association between the EPO-R and JAK2. Similarly the membrane proximal box 1 domain of gp130 was required for association with JAK2, but also associated with JAK1. This suggests that box 1 sequences are required for both JAK1 and JAK2 association with cytokine receptors but that sequences outside the box 1 domain regulate the specificity of JAK kinase association. IL-6 and CNTF, cytokines whose receptors utilize gp130, can stimulate the phosphorylation of JAK1, JAK2, and tyk2 depending on the cell line examined(Stahl and Yancopoulos, 1993). In contrast only JAK2 has been shown to associate with the EPO-R, in vivo(Miura, Nakamura et al., 1994). Our results demonstrated that the EPO-R and gp130 behave differently with regards JAK activation, in a heterologous cell line. Thus, receptor interactions with JAK kinases could be regulated in two ways; receptors for cytokines such as EPO encode the specificity of association within receptor protein sequence whereas JAK kinase interaction with receptors like gp130 must be influenced by cell-specific factors. It will be important to determine what structural features of the receptors regulate the specific interaction of JAK2 with the EPO receptor protein. Expression of exogenous EPO-R in some IL-3- and GM-CSF-dependent cell lines (BaF3, DA-1, 32D, and FDCP-1) confers upon these cells the capacity to proliferate in response to EPO(Youssoufian, Longmore et al., 1993). This is not the case for most IL-2-dependent cell lines (CTLL-2, HT-2)(Yamamura, Kageyama et al., 1992). Similarly, studies of the biochemical events following ligand binding to members of the cytokine receptor family have suggested conservation of signal transduction mechanisms between some members of the cytokine receptor family(Youssoufian, Longmore et al., 1993)(Ihle, Witthuhn et al., 1994). One possible interpretation for these observations is that specificity of response to a growth factor is obtained at the level of receptor expression rather than at the level of signal transduction. Given the normally limited expression of the EPO-R, we were interested to determine if the EPO-R was capable of generating a proliferative and differentiative signal in cells normally responsive to the ligands of other members of the cytokine receptor family. Retroviral vectors expressing EPO-R(wt) and EPOR(R129C) were constructed, primary hematopoietic progenitor cells (d12.5 fetal liver and 5-fluorouracil treated adult bone marrow) were infected, and cultured in methylcellulose in the absence or presence of added EPO(McArthur, Longmore et al., 1995)(Pharr, Hankins et al., 1993). Table 1. The association of IAK2 or JAKl
with the EPO-R or gp130 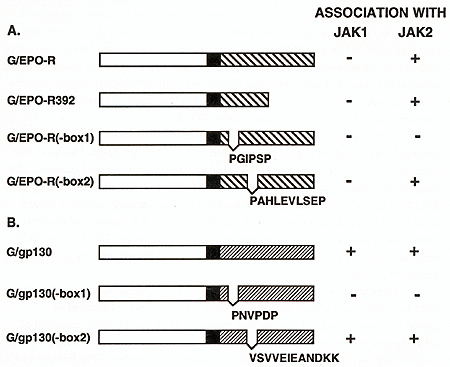
Table 2. Erythroid colony formation
in cultures of fetal liver cells induced to express EPO-R or EPO-R(R129C)

Hematopoietic cells at different stages in development are thought to have different complements of cytokine receptors. Whether the appearance of specific receptors initiates a particular developmental sequence is not known: Does the acquisition of a lineage-specific receptor induce differentiation? To address this question we utilized retroviral-mediated gene transfer to express the EPO-R in multilineage blast cell progenitors (Pharr, Ogawa et al., 1994) (Table 4). By DNA PCR we demonstrated that pluripotent blast cell clones could be infected with EPO-R(R129C)-expressing retroviruses. Blast cells and their progeny, CFU-GM and CFU-mix, express retrovirally derived EPO-R(R129C) as determined by PCR of cDNA prepared from these colonies (not shown). We observed no evidence that blast cells transduced with EPO-R(R129C) could induce erythroid differentiation, even following the addition of EPO. These results are consistent with in vivo experiemtns in which EPO has been shown to regulate the rate at which committed erythrocyte progenitors become erythroblasts. Table 3. Colony formation in cultures
of post 5-FU bone marrow cells induced to express EPO-R or EPO-R(R129C).

Table 4. Effect of EPO-R(R129C) on the
composition of mixed colonies derived from infected blast cells

Anagnostou, A., E. S. Lee, N. Kessimian, R. Levinson and M. Steiner .1990. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl. Acad. Sci. USA. 87: 5978-5982. D'Andrea, A. D., H. F. Lodish and G. G. Wong .1989. Expression cloning of the murine erythropoietin receptor. Cell 57: 277-285. Dai, C. H., S. B. Krantz and K. M. Zsebo .1991. Human burst-forming units erythroid need direct interaction with stem cell factor for further development. Blood. 78: 2493-2499. Ihle, I. N., B. A. Witthuhn, F. W. Quelle, K. Yamamoto, W. E. Thierfelder, B. Kreider and 0. Silvennoinen .1994. Signaling by the cytokine receptor superfamily: IAKs and STATs. Trends in Biochem. Sci. 19: 222-227. Keller, G., M. Kennedy, T. Papayannopoulou and M. V. Wiles .1993. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol. Cell. BioI. 13: 473-486. Krantz, S. B. .1991. Erythropoietin. Blood. 77: 419-434. Masuda, S., M. Nagao, K. Takahata, Y. Konishi, F. Gallyas, T. Tabira and R. Sasaki .1993. Functional erythropoietin receptor of the cells with neural characteristics. I. BioI. Chem. 268: 11208-11216. McArthur, G. A., G. D. Longmore, K. Klinger and G. R. Johnson .1995. Lineage restricted recruitment of immature hematopoietic progenitor cells in response to erythropoietin after transfection of normal hematopoietic cells with the erythropoietin receptor. Exp.Hematol..1995 McArthur, G. A., L. R. Rohrschneider and G. R. Johnson .1994. Induced expression of c-fms in normal hematopoietic cells shows evidence for both conservation and lineage restriction of signal transduction in response to M-CSF. Blood. 83: 972-980. Miura, 0., A. D. D'Andrea, D. Kabat and J. N. Ihle .1991. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol. Cell. BioI. 11: 4895-4902. Miura, 0., N. Nakamura, J. N. Ihle and A. Nobuo .1994. Erythropoietindependent association of phosphatidylinositol-s-kinase with tyrosinephosphorylated erythropoietin receptor. I. BioI. Chem. 269: 614-620. Murakami, M., M. Narakaki, M. Hibi, H. Yawata, K. Yasukawa, M. Hamaguchi, T. Taga and T. Kishimoto .1991. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc. Natl. Acad. Sci. USA. 88: 11349-11353. Ohashi, H., K. Maruyama, Y.-C. Liu and A. Yoshimura .1994. Ligandinduced activation of chimeric receptors between the erythropoietin receptor and receptor tyrosine kinases. Proc. Natl. Acad. Sci. (USA). 91: 158-162. Pharr, P. N., D. Hankins, A. Hofbauer, H. F. Lodish and G. Longmore .1993. Expression of a Constitutively Active Erythropoietin Receptor in Primary Hematopoietic Progenitors Abrogates Erythropoietin Dependence and Enhances CFU-E, BFU-E, and GM Progenitor Growth. Proc. Natl. Acad. Sci. USA. 90: 938-942. Pharr, P. N., M. Ogawa, A. Hofbauer and G. D. Longmore .1994. Expression of an activated erythropoietin receptor or a CSF-1 receptor by pluripotent progenitors enhances colony formation but does not induce differentiation. Proc. Natl. Acad. Sci. USA. 91: 7482-7486. Sawada, K., S. B. Krantz, C. H. Dai, S. T. Koury, S. T. Horn, A. D. Glick and C. I. Civin .1990. Purification of human blood burst forming units-erythroid and demonstration of the evolution of the erythropoietin receptor. L Cell. Physiol.l42: 219-230. Sawyer, S. T., S. B. Krantz and K.-I. Sawada .1989. Receptors for erythropoietin in mouse and human erythroid cells and placenta. Blood. 74: 103-109. Stahl, N. and G. D. Yancopoulos .1993. The alphas, betas, and kinases of cytokine receptor complexes. Cell 74: 587-590. Watowich, S. S., D. J. Hilton and H. F. Lodish .1994. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol. Cell. BioI. 14: 3535-3549. Watowich, S. S., A. Yoshimura, G. D. Longmore, D. Hilton, Y. Yoshimura and H. F. Lodish .1992. Homodimerization and constitutive activation of the erythropoietin receptor. Proc. Natl. Acad. Sci. USA. 89: 2140-2144. Witthuhn, B. A., F. W. Quelle, 0. Silvennoinen, T. Yi, B. Tang, 0. Miura and J. N. Ihle .1993. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell74: 227-236. Yamamura, Y., Y. Kageyama, T. Matuzaki, M. Noda and Y. Ikawa .1992. Distinct downstream signaling mechanisms between erythropoietin receptor and interleukin-2 receptor." EMBO I. 11: 4905-4915. Yoshimura, A., G. Longmore and H. F. Lodish .1990. "Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity ." Nature. 348: 647-649. Youssoufian, H., G. Longmore, D. Neumann, A. Yoshimura and H. Lodish. 1993. Structure, Function, and Activation of the erythropoietin receptor. Blood. 81(9): 2223-2236. |