|
Haematological Scientific Center Russian Academy of Medical Science Novosykovsky pr. 4a 125167 Moscow, Russia The hematopoietic system is created by hierarchical progression of commitment events starting from the primitive hematopoietic stem cell (HSC). By strict definition, the primitive HSC is the founder cell for the entire hematopoietic system, i.e. it is capable of long-term support of hematopoiesis both in vivo and in vitro. It is generally assumed that the primitive HSC has the capacity both for differentiation and self-renewal (Chertkov, Gurevitch, 1984) Nevertheless, crucial question about capacity of HSC for inexhaustible proliferation remains unanswered Many attempts to prove unlimited proliferative capacity of HSC by bone marrow cell passages always demonstrated the exhaustion of HSC potential after 3-5 transfers (Micklem et al, 1987). However, the technical reservation prevented from getting the conclusive evidence of inability of HSC to maintain itself. There is the other way to answer this intriguing question. if the founder cell has the capacity for self-renewal, hematopoiesis would be stochastic and many primitive HSCs would be functioning simultaneously. On the other hand, if the primitive HSC has no capacity to self-renew, hematopoiesis would be characterised by clonal succession. According to this model, primitive HSCs are used sequentially, one after the other, producing clones which replace each other ( Rosendaal, Adam, 1984). Reconstitution of irradiated mice with bone marrow cells carrying specific markers (HSC marked by retroviral integration) was used for characterisation of clonal succession (Williams et al, 1984; Lemischka et al, 1986; Snodgrass, Keller, 1987; Capel et al, 1989; Jordan, Lemischka, 1990). As a rule, after several months of " clonal fluctuations" , monooligoclonal hematopoiesis occurs and longevity of clone functioning can be as long as 2 5 years. In contrast, the results obtained with HSC without transduced DNA sequences suggested that hematopoiesis is polyclonal both in normal and reconstituted animals (Micklem et a11987; Harrisson et al, 1978 ). Differences in experimental results concerning the clonality of HSC may be due to the prestimulation of HSC during gene transfer procedure with pharmacological concentrations of hematopoietic growth factors. It is possible that all primitive HSC may be induced to proliferate by cytokines. Furthermore, progenitors with selective proliferative advantage could gradually replace all others, and after a time, only this one or several cell clones will be preserved (mono-oligoclonal hematopoiesis). In order to verify this hypothesis and to study the possible clonal succession of HSC, the next conditions must be realized: clonal composition of the hematopoietic system must be studied sequentially in the same reconstituted mouse; the method used must be very sensitive to detect a great number of simultaneously functioning clones during clonal selection; and the method of gene transfer without prestimulation with pharmacological concentration of cytokines should also be used. Here all these conditions were realized and hematopoietic cell clonal succession has been demonstrated conclusively. The proliferative potential and proliferative activity of more mature HSC, CFU-S-11 , in animals reconstituted both with treated by exogenous cytokines and untreated hematopoietic cells were characterized.
Mice. Male and female (C57BI/6 x DBA/2) F 1mice, 3-5 months-old
were used. Long-term hone marrow culture. Femoral bone marrow was
cultured in Fisher's medium supplemented with 20% of 2: 1 mixture
of horse and fetal bovine serum, 10high -6 M hydrocortisone , L-glutamine
and antibiotics as described (Chertkov et al, 1983). CFU-S-ll assay,
spleen repopulating ability and Hydroxyurea "suicide '1 of CFU-S-ll
Recipients in the spleen colony-forming (CFU-S) assay were exposed
to 1200 cGy 137 Cs irradiation (the dose rate 18 cGy/min, IPK irradiator).
The dose was delivered into two equal fractions, given 3 h apart
Irradiated female mice were injected i. v. with 1 to 4 x 10high
5 marrow from reconstituted mice. Individual macroscopic colonies
were isolated under dissection microscope 11 days later and used
both for DNA analysis and for determination of the selfrenewal capacity
(spleen repopulating ability /SRA/. number of daughter CFU-S-8 per
11-day colony). The colony cells (0.2 colony equivalent) were injected
into irradiated secondary recipients. The number of daughter colonies
were counted 8 days later and the number of colonies generated per
one CFU-S-11 was calculated. Proliferative activity of CFU-S-11
was determined by hydroxyurea (HU) suicide. Bone marrow cells from
reconstituted mice were incubated in RPMI 1640 media supplemented
with 5% fetal bovine serum for 2 hours at 37oC with and without
1 mg/ml HU, washed trice with Hanks' balanced salt solution (HESS)
and injected ( 1-2 x 10 high 5) into irradiated recipients Number
of spleen colonies was determined following 8 days. Suicide (Su)
was calculated by next equation: 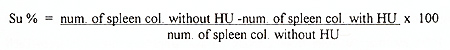
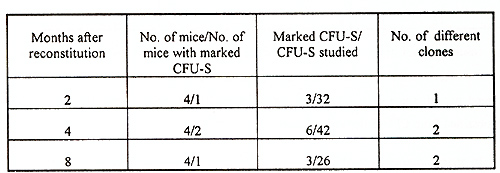
Table 1 Characteristics of mice reconstituted
with retrovirally marked bone marrow cells 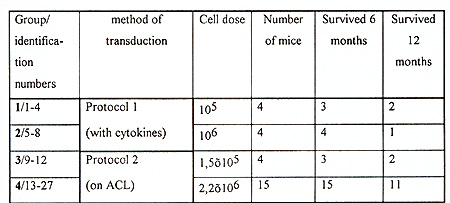
The efficiency of gene transfer into CFU-S-II was high, 52-83% of CFU-S derived colonies contained the human ADA cDNA (protocol I ). Without exogenous cytokines the efficiency of transduction was lower, only 18-32% of CFU-S derived colonies were marked (protocol 2). In many instances, several proviral copies per genome were integrated as demonstrated by Southern analysis (Fig. I). Transduction efficiency into primitive HSC also appeared to be good since, as seen in table 2, marked clones were still present in CFU-S-II from mice up to I year after reconstitution. Analysis of clonal composition of mice reconstituted with cytokine stimulated HSC revealed that 2 and 4 months after reconstitution the integration patterns varied between different colonies of the same mouse, implying that the progeny of many different clones were functioning simultaneously. From 23 colonies studied in first 4 months after reconstitution 22 had different unique markers (Table 2). Table 2. Clonal composition of the hematopoietic
system at different times after reconstitution 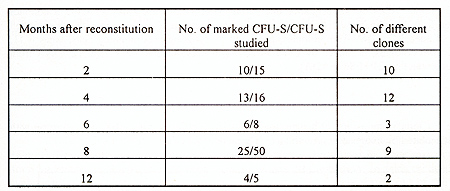 These results demonstrate that multiple clones are existing early after transplantation. However, oligo-monoclonal hematopoiesis appeared to become established over a more prolonged period, since only 1-2 clones were observed 6-12 months after reconstitution in DNA obtained from CFU-S derived colonies. In sharp contrast with these data were the results obtained with bone marrow cells prestimulated on ACLs without exogenous cytokines. No phase of polyclonal hematopoiesis was revealed in these mice (table 3). Efficiency of gene transfer into primitive HSC was lower and marked clones were observed only in 25-50% of reconstituted mice in each time interval studied However, even in first months after reconstitution only one or two marked clones were revealed No one of detected clones persisted more than 2-3 months. Disappearing clones were never observed again. As can be seen from table 3, the proportion of transduced cru-s is about 10-15% of CFU-S studied. These observations suggest that about one-tenth of all bone marrow CFU-S are derived from marked HSC 
 To confirm the donor origin of CFU-S we assessed the presence of y -chromosome sequences in DNA obtained from spleen colonies in all experiments. All colonies derived from reconstitution (Fig 2) Therefore, the procedure of gene transfer both with and without exogenous cytokines did not decrease the developmental potential of primitive HSC 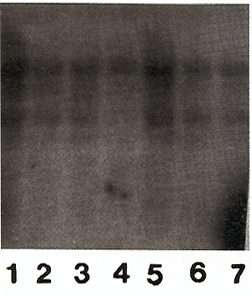
Proliferative activity of CFU-S was studied in mice 6 months following
reconstitution (Fig 3) The results were essentially the same in
groups reconstituted by bone marrow cells transduced both with and
without exogenous cytokines prestimulation. In mice reconstituted
with large cell inoculum suicide rate was about 10%, ie CFU-S were
mainly out of cell cycle as during steady-state hematopoiesis in
normal animals Mice reconstituted with low cell dose demonstrated
significant increase of suicide which was about 20% CFU-S with integrated
human ADA cDNA had 2-fold higher suicide rate than unmarked CFU-S.
However, because of low amount of studied marked colonies (9 from
42) the difference is insignificant. 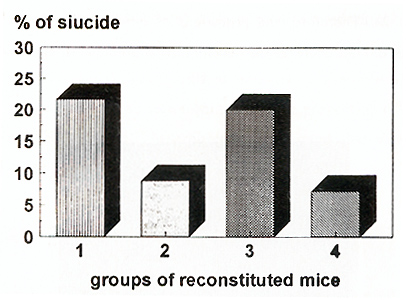
Spleen repopulating ability (SRA) of CFU-S-ll 6 and 12 months
after reconstitution was significantly decreased in all experimental
groups of mice (Fig. 4). The average number of daughter CFU-S per
11 day colony was 20-60 as compared with 150 for normal bone marrow
derived colony. In all mice reconstituted with cytokine prestimulated
cells SRA gradually decreased during 12 months. On the other hand,
two of five mice reconstituted with transduced cells without cytokine
prestimulation had higher SRA I year after reconstitution than that
after 6 months 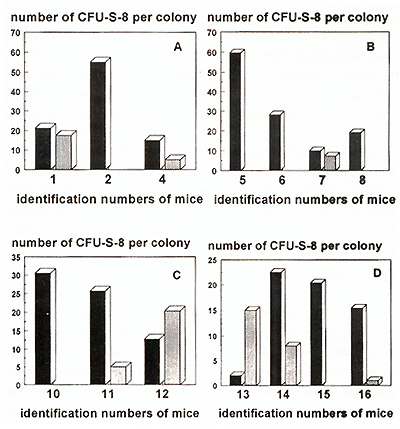
The results presented here demonstrate that the difference in the developmental fate of primitive HSC, measured with traditional methods (competitive repopulation assay, serial bone marrow passages et al), as compared with methods of retroviral gene transfer, could be explained by use of pharmacological doses of exogenous hematopoietic growth factors. A combination of cytokines (IL-6 + SCF) induced high efficiency gene transfer into HSC, what suggests that quiescent HSC had been stimulated for proliferation. Efficiency of gene transfer after more physiological prestimulation of HSC on irradiated ACL of long-term bone marrow culture was significantly lower, possibly because of low proportion of HSC that had been triggered in cell cycle In mice reconstituted with bone marrow cells prestimulated with exogenous growth factors, many marked clones appeared simultaneously. Two to 4 months after reconstitution, essentially each marked CFU-S represents an unique clone (22 of 23 colonies were represented by unique marker). Statistically, the results indicate that in the first months after transplantation 200-400 clones are functioning simultaneously (confidence interval, 95%) CFU-S is a progenitor with relatively low proliferative potential and it can produce differentiated progeny not more than 4 -5 weeks both in vivo (Bodine et al, 1991 ; Bodine et al, 1992) and in vitro (Chertkov et al, 1987) Therefore, during 2 -4 months after reconstitution the revealed marked clones are progeny of primitive HSC, rather than initial CFU-S Hence, many if not all primitive HSC are proliferative at least 4 months after transplantation During this time, selection of clonogenic progenitor(s) with proliferative advantages is possible, and this clone(s) can replace all others. As a result of such selection, all marked cells would have the same unique marker Indeed, 6 -12 months after reconstitution, hematopoiesis appeared to be oligo-monoclonal as shown here and by others (Rosendaal, Adam, 1984;Capelet al, 1989;Limet al, 1989) Essentially, different results were obtained after transplantation of cells not stimulated by exogenous cytokines The efficiency of gene transfer was significantly lower, however the system was suitable for the study of the hematopoietic clonal composition after reconstitution. No phase of polyclonality was observed in this model with use of an ACL for prestimulation. From the first months after transplantation only lor 2 marked clones have been observed. The longevity of marked clones existence was shorter than 2 -3 months because they were revealed only once during one year; analysis was performed each 2 -3 months. It appears that no stem cell selection took place and hematopoiesis was maintained by clonal succession. If appear simultaneously. Hence, the cause of peculiarity of results obtained with gene transfer technique is the use of pharmacological concentration of exogenous cytokines. If it is possible to avoid such stimulation of HSC and to use a more physiological gene transfer, the same clonal succession and limited proliferative potential of primitive HSC would be observed. The other evidence, that transduced foreign sequences do not change the proliferative potential of HSC, is the same SRA in marked and non-marked colonies The clonal succession of hematopoietic cells was also supported by the data about increase of SRA in 12 months as compared with 6 months in the group of mice reconstituted with the physiologically prestimulated cells (protocol 2). It is difficult to understand how can SRA increase in the progeny of the same primitive HSC In the case of clonal succession this effect could be explained by replacing the clones with low proliferating potential by clones which were less exhausted. The incapacity of primitive HSC to maintain itself is supported by the data obtained here and earlier (Chertkov et al, 1993 ), that after reconstitution with large cell innoculum the proliferative potential of progenitors, as measured by SRA, is higher than after reconstitution with low cell dose. Of course, this could not happen if the primitive HSC has unlimited proliferative potential Proliferative activity of CFU-S, as measured by HU suicide, was increased only in groups reconstituted with low dose of hematopoietic cells It is known, that clone size is bigger in animals transplanted with low doses of bone marrow cells ( Micklem et al, 1987). The data suggest that this increase of clone size is due at least partially by expansion of CFU-S at the expense of increased proliferative rate On the whole, the results support the clonal succession model of hematopoiesis and mortal nature of primitive HSC. The data demonstrate the possibility of using this model for the sequential study of the clonal composition of the hematopoietic system and primitive HSC developmental potential
The authors wish to thank Drs D. A. Williams and L.G.Brown for
generous gift of the GP+E86 (hADA) cell line and the y probe.
Bodine DM, Crosier PS, Clark SC (1991) Effects of hematopoietic growth factors on the survival of primitive stem cells in liquid suspension culture. Blood 78:914-920 Bodine DM, Orlic a, Birkett NC, Seidel NE, Zsebo KM (1992) Stem cell factor increases coJony-forming unit-spleen number in vitro in synergy with interleukin-6, and in vivo in SI/Sld mice as a single factor. Blood 79:913-920 Capel B, Hawley R, Covarrusias L, Hawley T, Mintz B (1989) Clonal contribution of small numbers of retrovirally marked hematopoietic stem cells engrafted in unirradiated neonatal W/W mice. Proc Natl Avcad Sci USA 86:4564-4567 Chertkov JL, Drize NI, Gurevitch OA, Udalov GA (1983) Hemopoietic stromal precursors in long-term culture of bone marrow I. Precursors characteristics, kinetics in culture, and dependence on quality of donor hemopoietic cells in chimeras. Exp Hematol 11:231-242 Chertkov JL, Gurevitch OA (1984) Hematopoietic stem cell and its microenvironment. Moscow. Meditzina Chertkov JL, arize NI, aeryugina El, Udalov GA (1987) Individual clones of hemopoietic cells in murine long-term bone marrow cultures Leukemia 1: 491-496 Chertkov II, Iiang S, Lutton JD, Harrison I, Levere Ra, Tiefenthaler M, Abracham NG (1993) The hematopoietic stromal microenvironment promotes retrovirus-mediated gene transfer into hematopoietic stem cells. Stem Cells II 218-227 Jourdan CT, Lemischka IR ( 1990) Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev 4.220 Harrison DE, Astle CM, aelaittre IA (1978) Loss of proliferative capacity in immunohemopoietic stem cells cause by serial transplantation rather then ageing. I Exp Med 147:1526-1531 Lemischka IR, Raulet OH, Mulligan RC ( 1986) Developmental potential and dynamic behaviour of hematopoietic stem cells. Cell 45.917-923 Lim B, Apperley JF, Orkin SH, Williams DA ( 1989) Long-term expression of adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells Proc Nat Acad Sci USA 86 8892-8895 Luskey BD, Rosenblatt M, Zsebo K, Williams DA (1992) Stem cell factor, interleukin3, and interleukin-6 promote retroviral-mediated gene transfer into murine hematopoietic stem cells Blood 80:396-405 Micklem HS, Lennon JE, Amsell 10, Gray RA (1987) Minbers and dispersion of repopulating cell clones in radiation chimeras as a function of injected cell dose. Exp Hematol 15:251-257 Rosendaal M, Adam J (1984) Haemopoiesis by clonal succession? Blood Cells 10:473- 485 Snodgrass R, Keller G ( 1987) Clonal fluctuation within the hematopoietic system in mice reconstituted with retrovirus-infected cells EMBO J 6:3955-3961 Williams DA, Lemischka IR Nathan DG, Mulligan RC (1984) Introduction of new genetic material into pluripotent stem cells of the mouse Nature 310:476-482
Developmental fate of retrovirally marked hematopoietic stem cells (HSC) critically depends on the use of pharmacological concentration of growth factors during gene transfer procedure Cytokines (interleukin-6 plus stem cell factor) stimulated HSCs proliferate at least 4 months after reconstitution. At this time hundred of hematopoietic clones are exist simultaneously. Later as a result of clonal selection only oligo-clonal hematopoiesis was detected. Vice verse, after more physiological prestimulation of HSC on irradiated adherent cell layer of long-term bone marrow culture no phase of polyclonal hematopoiesis has been observed Hematopoiesis was according to clonal succession model and replacing each other hematopoietic cell clones were revealed The longevity of clone existence was not longer than 2-3 months The proliferative activity of CFU-S was higher in reconstituted with small dose of bone marrow cells mice That could be connected with enlargement of individual clone size in these animals The results support the clonal succession model of hematopoiesis and incapacity of primitive HSC to maintain itself The data demonstrate the possibility of using this model for the sequential study of the clonal composition of the hematopoietic system and primitive HSC developmental potential |