|
* Supported in part by NIH grants and the 4 E Leukemia
Fund in memory of Marilyn Levine and Irvin Epstein, the Concern
Foundation, Parker Hughes Fund, & Realtors of Real Estate Division.
Dr. H. Phillip Koeffler is a member of the Jonsson Comprehensive
Cancer Center . Introduction Life span of mature blood cells is ephemeral, requiring hematopoiesis throughout life. A complex network of hematopoietic progenitor cells and cytokines maintain an enormous daily production of granulocytes, monocytes, erythrocytes, platelets, and lymphocytes. This population of hematopoietic cells must be able to respond rapidly to changing needs such as bleeding, infections, cancer, or exposure to cytotoxic agents. Colony stimulating factors (CSFs) are a family of glycoproteins that promote growth and differentiation of hematopoietic progenitor cells and also enhance the function of the mature blood cells. production of CSFs is under tight control since either their over- or underproduction will result in dysregulation of hematopoiesis. Proliferation of hematopoietic progenitor cells require the continuous presence of these factors. A variety of cells including nonhematopoietic cells such as fibroblasts, endothelial cells, and smooth muscle cells are capable of producing many kinds of CSFs. This chapter will describe cells that make CSFs and the mechanisms involved in this production.
Mesenchymal cells originate from either mesoderm or ectoderm. Three
major cells that compose the mesenchymal tissues include fibroblasts,
vascular endothelial cells, and smooth muscle cells. Fibroblasts
provide the scaffolding required for cellular organization; this
extracellular matrix is required for tissue cohesion [39, 40]. These
cells are not functionally effete; they produce CSFs and a variety
of cytokines. They playa major role in the response to tissue injury,
being the primary cells involved in tissue repair . These cells
respond to interleukin-1 (IL1) and tumor necrosis factor (TNF) by
proliferation, synthesis, and assembly of collagen [18]. Fibroblasts
in the bone marrow may function as part of the microenvironment
[15, 21]. Pluznik and Sachs originally showed that fibroblasts could
produce CSF [36]; somewhat more recently endothelial cells and smooth
muscle cells were found to be capable of stimulating granulopoiesis
[25, 35]. Studies have not shown definitely that, in vivo, these
cells constitutively make CSF. Our preliminary studies suggest that
embryonic and adult lung fibroblasts produce very low amounts. Sustained
myelopoiesis in long-term culture of bone marrow cells requires
the presence of stromal cells composed of a complex network of cell
types, including fibroblasts and endothelial cells [16]. These cells
produce low levels of hematopoietic growth factors. 
Physiological Stimulators of CSF Production Bagby et al. [4, 5] initially noted that macrophages exposed to
lipopolysaccharide produced factors that stimulated both endothelial
cells and fibroblasts to produce CSFs. Several years later, we [31]
found that TNF-a, one of the products of macrophages, was able to
stimulate fibroblasts, endothelial cells, and smooth muscle cells
to produce CSFs. At the same time, IL-l was noted to increase synthesis
of CSFs in the same cells [6, 8, 27, 45]. Further studies have shown
that mesenchymal cells cultured with either TNF or IL-l b produced
macrophage CSF (M-CSF) [1, 24], as well as IL-l b and IL-6 [2, 28,
42]. We have noted that messenger ribonucleic acid (mRNA) for each
of these growth factors is produced in a coordinate fashion after
mesenchymal cells are stimulated by either TNF or IL-l b. Lymphotoxin,
which is produced by activated lymphocytes and has peptide homology
to TNF, can also stimulate mesenchymal cells to produce CSFs, although
the potency of this cytokine may be less than TNF (Fig. 1) [1, 9].
TNF-a and IL-l bare made in abundant amounts by activated macrophages
[17, 34]; lymphotoxin is mostly synthesized by activated lymphocytes.
A number of conditions, including bacterial invasion, are known
to stimulate these cells to synthesize TNF, IL-l, and lymphotoxin,
which can enhance CSF production by mesenchymal cells. This inter
communication of cells results in a cascade of synthesis of cy to
kine in the region ofinflammation, such as sites of bacterial and
viral infections, rheumatoid arthritis, and some collagen vascular
disorders. Steady state hematopoiesis in the bone marrow perhaps
is in part regulated by the constant, short-range production of
cytokines synthesized by mesenchymal cells, macrophages, and lymphocytes.

Mesenchymal cells have detectable levels of CSF mRNA within 30-60 min of exposure to TNF [27]. This stimulation by TNF can occur in the absence of new protein synthesis [27]. Previous studies have shown that protein kinase C activators can increase accumulation of CSFs in fibroblasts (Fig. 2) [1, 27]. Depletion of protein kinase C activity by prolonged exposure of fibroblasts to 12-0-tetradecanoylphorbo113-acetate (TPA) blocks the accumulation of GMCSF RNA by TP A but does not affect the accumulation of this RNA induced by TNF [41]. This result suggests that the effect of TNF is independent from protein kinase C activation. Further studies of TNF showed that it can cause the alkalinization of mesenchymal cell; this probably occurs through stimulation of the Na+/H + antiporter [43]. Amiloride blocks this alkalinization, but does not block accumulation ofGMCSF mRNA. Taken together, our experi ments provide strong evidence that TNF does not mediate its action through either PKC or Na+/H+ antiporter. U sing an array of agonist and antagonist, we found that those agents that increase levels of intracellular Ca² + and K + also increase levels of CSF mRNA [43]. Increase of K + levels may stimulate the Ca²+/K+ pump causing increased levels of cytosolic Ca ² + .In addition, we found that NaF in the presence of Al³ + is a potent stimulator of levels of CSF mRNA. This stimulation cannot be blocked by pertussis toxin suggesting that NaF/Al³+ may be enhancing the activity ofG-binding proteins that are insensitive to the action of pertussis toxin. This observation is consistent with preliminary data suggesting that transformation of mesenchymal cells by transfection of activated H-ras can lead to their increased expression of GM-CSF mRNA. Some tumors are able to synthesize CSF constitutively and patients with these tumors often have peripheral blood leukocytosis. We examined cell lines from tumors that produced CSFs; these tumors were associated with leukocytosis in the patients (H. Ross and H. P. Koeffler, in preparation). Cells of each expressed high levels G M -, G-, and M-CSF mRNAs as well as IL-l and IL-6 mRNAs. Furthermore, the stability of mRNA coding for each of these growth factors was 10 to 20-fold greater than that in nontransformed cells. The tumors have well-defined oncogene alterations that may be closely associated with inappropriate stability of normally transiently expressed genes.
Macrophages are pivotal in inflammation and immunity. In the 1970's, monocytes/macrophages were found to produce CSF [12, 20]. Further studies have found that human monocytes/macrophages from many tissues produce predominantly G- and M-CSF, as well as IL1, IL-6, and TNF, but synthesize very little GM-CSF. However, other studies found that human monocytes/macrophages accumulate GM-CSF when exposed to lipopolysaccharide, fetal calf serum, or thioglycollate, or when cells phagocytose and adhere in the presence of fibronectin [38]. Resting macrophages produce little CSF, but their synthesis of CSF markedly increase with activation after exposure to a variety ofphysiologically relevant agents including TNF , interferon-r (IFN-r), GM-CSF, IL-3, IL1, and endotoxin. Besides producing MCSF, IL-l, and TNF, these cells have receptors for cytokines, suggesting that under certain circumustances these cells might develop an autocrine stimulation which might foster inflammation. This inflammation may be either salutary (e.g., bacterial infections) or detrimental (e.g., rheumatoid arthritis). Nuclear runon transcription assay and half-life studies showed that the induction of Gand M-CSF genes is due to mRNA stabilization [19].
Granulocytes share a number of common properties with monocytes, including phagocytic activity, similar membrane receptors, and a common progenitor cell. They are relatively short-lived, nondividing cells, which often are considered to have little biosynthetic capacity. However, as early as 1948, granulocytes were known to release endogenous pyrogen [7]. Recent studies have demonstrated that granulocytes can be induced to accumulate mRNA coding for G- and MCSF, IL-l, and TNF after exposure to GM-CSF [30]. These cells also produce a number of other proteins such as plasminogen activator [22], FOS [23], and IL1 [29]. These findings suggest that neutrophils may be involved in the regulation of hematopoietic growth factors.
Cline and Golde [13] first showed that human lymphocytes in vitro produce significant CSF; these cells especially synthesize large amounts when stimulated with lectin or antigenic stimulation. CSF can be synthesized by both CD4+ and CD 8 + lymphocytes; the former are the most potent producers of cytokines. T lymphocytes can produce all the interleukins and GM-CSF. The cells lack the ability to secrete G- and M-CSF suggesting that transregulatory proteins may be different in mesenchymal cells and T lymphocytes, and those that regulate Gand M-CSF production possibly are different from those that control G M -CSF . A recent study showed that mRNA for M-CSF can accumulate in natural killer cells stimulated with IL-2 and CD 16 ligands [14]. Only T lymphocytes secrete IL-3 in the human system [44]. The in vivo importance of production of CSFs by T lymphocytes is unclear . They are present in small but significant numbers in bone marrow, allowing them to interact closely with hematopoietic progenitor cells by releasing growth factors. A role of lymphocytes in regulating normal hematopoiesis is indirectly suggested by alternation of hematopoiesis with alternation of subsets of lymphocytes. Nevertheless, children with congenital deficiencies of T lymphocytes appear to have fairly normal myeloid hematopoiesis, suggesting that other sources of CSFs can compensate for a lack of T lymphocytes.
In the resting state, both mesenchymal cells and macrophages transcribe cytokines, but do not accumulate these mRNAs (Table 1). With stimulation, cy to kine mRNA accumulates in macrophages and mesenchymal cells as well as in I lymphocytes. Maximal mRNA accumulation occurs after 2-8 h of stimulation in all three cell types. The constellation of cytokines produced by each of these cells differs. F or example, G- and M-CSF mRNA can be synthesized by mesenchymal cells and macrophage, but not by T lymphocytes; GM-CSF mRNA is produced predominantly by T lymphocytes and mesenchymal cells, but little is synthesized by human macrophages. Many of the same signals ofCSF production are operative in two or three of the cell types including IL-l, TNF, agents that increase intracellular calcium levels, endotoxin, and stimulators of protein kinase C. T lymphocytes are unique for several reasons. Studies suggest that they require two signals for CSF production instead of one, such as lectin plus phorbol ester, or calcium ionophore plus phorbol ester. In contrast, only one is probably required for macrophages and mesenchymal cells. T lymphocytes are also unique in another manner; these cells can be stimulated by special antigens, in the presence of an antigen presenting cell, to produce CSFs.
We constructed a promoter-reporter gene construct containing various regions of the GM-CSF gene 5' to the start site of transcription. These were transfected into fibroblasts and stimulated with either TNF or IL-l. These constructs showed no enhancement of reporter-gene activity. In contrast, protein kinase C activators markedly increased levels of the reporter-gene [33]. These are consistent with our notion that TNF and IL-l do not have a major effect on transcription of CSF but modulate post-transcriptionally levels of CSFs. On the other hand, protein kinase C activators stimulate both transcription as well as stabilization of these CSF mRNAs in each of the cell types. Promoter sequences encompassed by -53 to the start site for transcription of the GM-CSF gene are required to stimulate transcription by protein kinase C activators in mensenchymal cells and lymphocytes [11]. RNA of most cytokines including GMCSF have a short half-life. Stabilization Of short-lived mRNAs playa pivotal role in the accumulation of cytokines in each of the cell types. AU-rich sequences have been found in the 3' untranslated regions of most of the genes coding for these short-lived cytokines and oncogenes [10, 37]. We found that TNF, IL-1b, phorbol diesters, NaF, and cycloheximide enhance expression of GM-CSF RNA through stabilization (Fig. 3) [1, 27, 41]. We transfected into fibroblasts constructs containing an AT -rich sequence from GM-CSF gene placed into the 3' untranslated region of the reported gene [3]. These transfected cells were stimulated with TPA, cycloheximide, NaF, TNF, or IL-1b. TPA, NaF, and CHX required an AT -rich sequence for stabilization of the reporter gene. On the other hand, a reporter gene containing the AT -rich sequence does not respond to either TNF or IL-1 b. These experiments suggest that TNF and IL-1 b stabilize GM-CSF RNA independent of these AU sequences and that different mechanisms are used by various agents to stabilize GM-CSF RNA. Table 1. Regulation of CSF  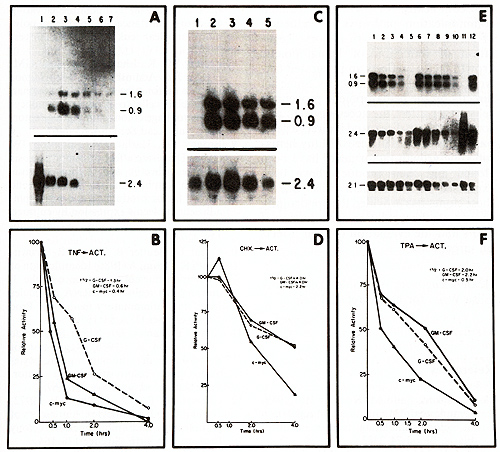
Conclusion Hematopoietic cells are produced and destroyed continuously under precise control. This chapter begins to illustrate the complexity of cytokine-mediated communication pathways in induction of hematopoietic growth factors. Regulation of induction of hematopoietic growth factors reflects an integrated network of bioregulator molecules. Some act directly on the hematopoietic progenitor cells; other affect the accessory cell; and some have both direct and indirect affects on the hematopoietic cells. Many hematopoietic growth factor genes have been cloned and their products have been expressed in mammalian cells. U se of these clones has provided the opportunity to evaluate the regulation of expression of CSFs and the evaluation of their affects on target cells.
We would like to thank Elisa Weiss for her secretarial help.
1. Akashi M, Saito M, Koeffler HP (1989) Lymphotoxin: stimulation and regulation of colony-stimulating factors in fibroblasts. Blood 74:2383 2. Akashi M, Loussararian AH, Adelman DC, Saito M, Koeffler HP (1990) Role of lymphotoxin in expression of IL-6 in human fibroblasts: stimulation and regulation. 1 Clin Invest 85: 121 3. Akashi M, Shaw a, Gross M, Saito M, Koeffler HP (1991) Accumulation ofaMCSF RNA by TNF and IL-l is regulated independently from AU-rich regions 4. Bagby ac, McCall E, Bergstrom KA, Burger D (1983) A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood 62: 663 5. Bagby ac, McCall E, Layman DL (1983) Regulation of colony-stimulating activity production: interactions of fibroblasts, mononuclear phagocytes and lactoferrin. 1 Clin Invest 71: 340 6. Bagby ac, Dinarello CA, Wallace P, Wagner C, Hefneider S, McCall RE (1986) Interleukin-l stimulates granulocyte and macrophage colony-stimulating activity release by endothelial cells. 1 Clin Invest 78:1316 7. Beeson PB (1948) Temperature elevating effect of a substance obtained from polymorphonuclear leukocytes. 1 Clin Invest 27:524 8. Broudy VC, Kaushansky K, Sehgal aM, Harlan lM, Adamson lW (1986) Tumor necrosis factor type A stimulates human endothelial cells to produce granulocyte/macrophage colony stimulating factor. Proc Natl Acad Sci USA 83: 7467 9. Broudy VC, Harlan lM, Adamson lW (1987) Disparate effect of tumor necrosis factor-a/cachectin and tumor necrosis factor-b/lymphotoxin on hematopoietic growth factor production and neutrophil adhesion molecule expression by cultured human endothelial cells. 1 Immunol 138:4298 10. Caput D, Beutler B, Thayer R, BrownSmith S, Cerami A ( 1986) Identification of a common nucleotide sequence in the 3' untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA 83: 1670 11. Chan lY, Slamon Dl, Nimer SD, aolde DW, aasson lC (1986) Regulation of expression of human granulocytemacrophage colony-stimulating factor. Proc Natl Acad Sci USA 83: 8669 12. Chervenick PA, LoBuglio AF (1972) Human bloods monocytes, stimulators of granulocyte and mononuclear colony formation in vitro. Science 178: 164 13. Cline Ml, aolde DW (1974) Production of colony-stimulating activity by lymphocytes. Nature 248: 703 14. Cuturi MC, Anegon I, Sherman F, Loudon R, Clark S, Perussia B, Trinchieri a (1989) Production of hematopoietic colony-stimulating factors by human natural killer cells. 1 Exp Med 169: 569 16. Dexter TM, Allen TD, Lajtha La (1977) Conditions controlling the proliferation of hematopoietic stem cells in vitro. 1 Cell Physiol 91: 335 15. Dexter TM (1982) Stromal cell associated hematopoiesis. 1 Cell Physiol [Suppl] 1: 87 17. Dinarello CA (1984) Interleukin-l. Rev Infect Dis 6: 51 18. Dinarello CA (1985) An update human interleukin-l: from function in relation to granulopoiesis in aplastic anemia. 1 Clin Immuno15:237 19. Ernst Tl, Ritchie AR, Demetri aD, Griffin lD (1989) Regulation of granulocyteand mononcyte-colony-stimulating factor mRNA levels in human blood monocytes is mediated transcriptional 264:5700 20. Golde DW, Cline MJ (1972) Identification of colony-stimulating cells in human peripheral blood. J Clin Invest 51: 2981 21. Gordon MY, Gordon-Smith EC (1983) Bone marrow fibroblasts function in relation to granulopoiesis in aplastic anemia. Br J Haematol 53: 483 22. Grancli-Piperno A, Vassalli JD, Reich E (1977) Secretion of plasminogen activator by human polymorphonuclear leukocytes. J Exp Med 146:1693 23. Heidorn K, Kreipe H, Radzun HJ, Muller R, Parwaresch MR (1987) The protooncogene c-fos is transcriptionally active in normal human granulocytes. Blood 70:456 24. Kaushansky K, Broudy VC, Harlan JM, Adamson JW (1988) Tumor necrosis factor-alpha and tumor necrosis factorbeta (lymphotoxin) stimulate the production of granulocyte-macrophage colonystimulating factor, and IL-1 in vivo. J Immunol141 :3410 25. Knudtzon S, Mortenson BT (1975) Growth stimulation of human bone marrow cells in agar culture by vascular cells. Blood 46:937 26. Koeffler HP, Gasson J, Ranyard J, Souza L, Shepherd M, Munker R (1987) Recombinant human TNFa stimulates production of granulocyte colony-stimulating factor. Blood 70: 55 27. Koeffler HP, Gasson J, Tobler A (1988) Transcriptional and post transcriptional modulation of colony-stimulating factor and other agents. Mol Cell BioI 8: 3423 28. Kohase M, Heriksen-DeStefano D, May LT, Vilcek J, Sehgal PB (1986) Induction of beta-2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell 45: 659 29. Lindemann A, Riedel D, Oster W, Meuer SC, Blohm D, Mertelsmann RH, Herrmann F (1988) Granulocytes/macrophage colony-stimulating factor induces interleukin-1 production by human polymorphonuclear neutrophils. J Immunol 140:873 30. Lindemann A, Riedel D, Oster W, ZieglerHeibrock HWL, Mertelsmann RH, Herrmann F (1989) Granulocyte/macrophage colony-stimulating factor induces cytokine secretion by human polymorphonuclear leukocytes. J Clin Invest 83: 1308 31. Munker R, Gasson J, Ogawa M, Koeffler HP (1986) Recombinant human TNF induces production of granulocytemonocyte colony-stimulating factor. Nature 323: 79 32. Nawroth PP, Bank I, Handley D, Cassimeris J, Chess L, Stem D (1986) Tumor necrosis factor /cachectin interacts with endothelial cell receptors to induce release of interleukin-1. J Exp Med 163: 1363 33. Nimer S, Gates MJ, Koeffler HP, Gasson J (1989) Multiple mechanisms control the expression of granulocyte-macrophage colony-stimulating factors by human fibroblasts. J Immunol143 :2374 34. Oliff A (1988) The role of tumor necrosis factor ( cachectin) in cachexia. Cell 54: 141 35. Quesenberry PJ, Gimbron MA (1980) Vascular endothelium as a regulator of granulopoiesis: production of colonystimulating activity by cultured human endothelial cells. Blood 56: 1060 36. Pluznik DH, Sachs L (1965) Cells in tissue culture. J Cell Comp Physiol 66:319 37. ShawG, Kamen R (1986) A conserved AU sequence from 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46:659 38. Thorens B, Mermod JJ, Vassalli P (1987) Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macro phages through post transcriptional regulation. Cell 48: 671 39. Umetsu DT, Katzen D, Labara HH, Geha RS (1986) Antigen presentation by human dermal fibroblasts: activation of resting T lymphocytes. J Immunol 136: 440 40. Whiteside TL, Worral RK, Prince RK, Buckingham RB, Rodnan GP (1985) Soluble mediators from mononuclear cells increase the synthesis of glycosaminoglycan by dermal fibroblasts cultures derived from normal subjects and progressive systemic sclerosis patients. Arthritis Rheum 28: 188 41. Yamato K, EI-Hajjaoui Z, Kuo JF, Koeffler HP (1989) Granulocyte-macrophage colony stimulating factor: signals for its mRNA accumulation. Blood 74: 1314 42. Yamato K, EI-Hajjaoui Z, Koeffler HP (1989) Regulation of levels ofIL-1 mRNA in human fibroblasts. J Cell Physiol 139:610 43. Yamato K, Kurtz I, EI-Hajjaoui Z, Koeffler HP (1991) Tumor necrosis factor stimulates N a+ /H + antiporter in hu man fibroblasts: dissociation between intracellular alkalinization and cy to kine mRNA accumulation 44. Yang YC, Clarletta AB, Temple PA, Chung MP, Kovacic S, Witek-Giannoti JS, Leaty AC, Kriz R, Donahue RE, Wong GG, Clark SC (1986) Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell 47: 3 45. Zucali JR, Dinarello CA, Oblon Dl, Gross MA, Anderson L, Weiner RS (1986) Interleukin-1 stimulates fibroblasts to produce granulocyte-macrophage colonystimulating activity and prostaglandin E 2. J Clin Invest 77: 1857 |