|
1 Paterson Institute for Cancer Research, Christie
Hospital and Holt Radium Institute, Manchester, M20 9BX, United
Kingdom
It is widely accepted that most naturally occurring leukemias are monoclonally derived from multipotent stem cells [5- 7, 17], but the genetic changes leading to their transformation are poorly understood. A useful system in which to study the various processes occurring during leukemogenesis is offered by nonleukemic, multipotent stem cell lines (FDCPmix) established from murine long-term marrow cultures [20]. These cells grow continuously in vitro in the presence of 11-3, but they can also be induced to differentiate into mature granulocytes, macrophages, erythrocytes, and occasionally megakaryocytes, eosinophils, and mast cells by serum factors [20] or in association with marrow stromal cells [20] or certain embryonic mesenchymal cell lines [18]. Recent data have shown that hematopoietic growth-fac tor-dependent progenitor cell lines ac quire growth-factor-independent growth and tumorigenicity when they are infected with retroviral vectors containing genes coding for 11-3 or GM-CSF [10, 11] .However , these studies have been restricted to cell lines which are blocked in differentiation and may therefore not reflect the alterations that occur in stem cells during leukemogenesis. To determine the effects of aberrant expression of 11-3 in differentiation-inducible stem cells we infected FDCPmix cells with a selectable retroviral vector carrying the cDNA of 11-3.
A cDNA clone of 11-3 (kindly provided by N. Gough, Melbourne) was subcloned into the MPSV -based M3neo vector [9, 10] and used for transfections into the amphotropic helper cell line, PA 317 [16], to produce infectious M3 MuV particles. Cell clone psi2 mos- 1 no.4 con taining the neo MPSV mas deletion vector [22] was used to infect PA 317 in order to obtain amphotropic pseudotypes necessary for the infection of the ecotropicvirus producing FDCPmix cell lines [23]. Cell clones with titres of 10 high 3-10 high 5 for MuVand 10 high 5-10 high 8 GTU for mos-l and with intact proviral genomes were used for co-cultivation experiments.
Virus-producing cell lines were kept in minimal essential medium supplemented with 10% fetal calf serum. Hematopoietic cell lines were maintained in Iscove's modified Dulbecco's medium, supplemented with 20% horse serum and Wehi 3BD-conditioned medium (WEHI CM) as a source of multi-CSF (I1-3) at a concentration that stimulated optimal cell growth.
10 high 5 FDCPmix cells were inoculated onto subconfluent irradiated (20 Gy) virusproducer cell lines. Various FDCPmix cell lines were used. Two days later, the loosely adherent cells and cells in suspension were harvested, washed, and resuspended at about 10 high 5 cells/ml. After 2 days of culture, G418 was added to a final concentration of 1 mg/ml and the cells were subcultured as appropriate. Non-virus-infected cells died within 7 days but cells which had been co-cultured on the M3MuV and M3neo-producer cell lines continued to proliferate in the presence of the G418. About 2 weeks after selection with G418, the cells were cloned in soft agar in the presence of I1-3, and individual colonies were isolated and expanded from three different FDCPmix cell lines.
M3-MuV infected cells (10 high 6) were washed twice to remove residual I1-3 and incubated without WEHI-CM for 48 h. The supernatant was used as such or concentrated tenfold via Amicon filtration (exclusion mol. wt. < 10000), dialyzed, and tested for stimulatory activity on indicator cell lines by determining [³H] thymidine incorporation. Half-maximal stimulation of FDCP2 cells by either WEHI-CM or recombinant murine I1-3 was defined as 50 U /ml.
One of the clones of FDCPmix infected with M3MuV was grown at high density in the absence of 11-3. Cells were washed twice in medium without I1-3 and plated, 1 x 10 high 4 cells/well, into 96-well plates. Dilutions of the antiserum of preimmune rabbit serum ranged from 1: 20 to : 10240 final. 30 h after initiation, 0.5 µCi [³³] thymidine was added for 14 h. Cells were harvested onto filters, using a cell harvester (Titertec), and counted.
10³ control uninfected cells and cells infected with M3neo virus alone or M3MuV were plated in soft agar in culture conditions which allow the expression of multiple hematopoietic lineages [19]. Individual colonies were isolated after 10 days of growth, cytospin preparations made, and the cells stained with benzidine plus May-Grunwald Giemsa. At least 30 colonies were examined from each group.
Stromal cell cultures derived from bone marrow were irradiated [20, 21] and used as a supportive stroma for the growth of the FDCPmix cells, either uninfected, or M3MuV or M3neo infected. Between 2 x 10 high 6 and 10 high7 FDCPmix cells were cocultured with the marrow stromal cells. At various times after seeding of marrow stroma by the FDCPmix cells, cytospin preparations of the nonadherent cells were performed and the cells stained with May-Grunwald Giemsa.
After two washes, aliquots of 5 x 10 high 5 FDCPmix cells, infected with either M3MuV or M3Neo, were inoculated into each diffusion chamber (DC). These were then inserted intraperitoneally into male CBA mice. After 7 days of culture the animals were killed; the chambers were removed and shaken for 40 min in a 0.5% Pronase solution (Merck). The resulting cell suspensions were counted for the total number of nucleated cells. Cytospin preparations were made and the cells were classified according to morphological criteria [12].
Cellular DNA was isolated and restricted by standard techniques and separated on agarose gels. Total RNA was isolated as previously described [2] and transferred to Gene Screen Plus (NEN) after denaturation with glyoxal and dimethylsulfoxide and electrophoresis through agarose gels [14]. Nucleic acids were transferred to Gene Screen Plus (NEN) and hybridized under the conditions recommended by the manufacturer, with probes labeled as previously described [4]. Probes used for analysis included an EcoRI-NcoI fragment of pMu21A containing the 11-3 cDNA clone (N. Gough, unpublished), the BglIII-BamHI fragment of pAG60 containing the coding region of the neo gene [3], and the PY80B probe specific for the murine Y -chromosome [1].
Uninfected and A4/M3neo cells (cultured with 11-3) and A4/M3MuV cells (cultured without 11-3) were suspended in Fischer's medium at an appropriate cell concentration. The cells were injected i. v. in syngeneic B6D2Fl mice that had received 10 Gy, prior to inoculation of the cells.
I. Virus Integration, Gene Expression, and Il-3 Secretion Following infection and selection in liquid culture the cells were cloned in soft agar in the presence of 0418. Individual clones were isolated and cultured for further analysis. Analysis of the virus insertion sites revealed that the resulting cell lines were monoclonal ( data not shown). The I1-3 gene was expressed in the MuV infected FDCPmix cells, as shown by Northern analysis (data not shown). Conditioned medium of the M3MuV-infected cells growing in the absence of Il-3 contained between 4 and 50 units of Il-3 activity per ml.
All M3MuV infected cell lines could grow in high density without
Il-3, whereas the uninfected FDCPmix cells and the M3neo-infected
cells died in the absence of growth factor. Cloning of FDCPmix M3MuV-infected
cells in soft agar resulted in nonlinear, density-dependent growth
in the absence ofI1-3 and in nearly linear growth in the presence
of I1-3 (Fig. 1). Growth of the M3MuV-infected cells could be blocked
by neutralizing antisera to I1-3 (Fig. 2). 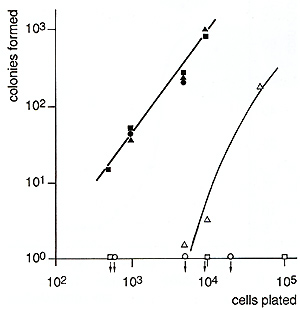
Table I. Colony formation by control and
infected FDCPmix cells 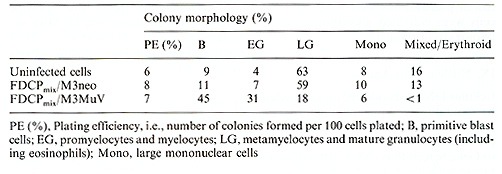 Table 2. In vivo administration of FDCPmix cells 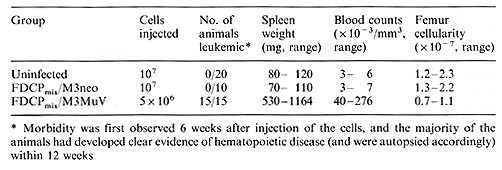
M3MuV-infected stem cell lines retained their capacity to undergo
differentiation in response to serum factors or marrow stromal cells.
In the mixed colony assay the plating efficiency was unaltered and
the colonies produced contained maturing granulocytes and macrophages
(Table 1). However, erythroid cells were rarely seen, and the balance
between immature and mature granulocytes was changed in favor of
immature cells (Table 1, Fig. 3). The same was true when the cells
were co-cultured with marrow stromal cells (data not shown). Culture
of M3MuV-infected FDCPmix cells in vivo in the DC led to an increase
of immature and mature granulocytes and macrophages similar to the
in vitro observations. In addition, erythroblasts were also found
in the DC (data not shown). 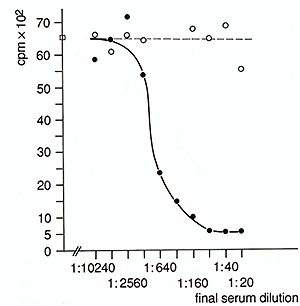
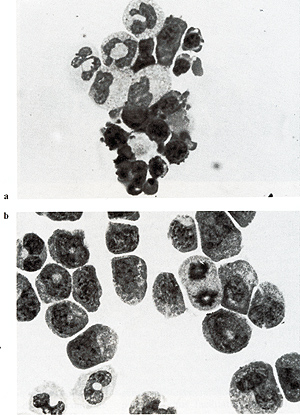 Fig. 3 a, b. Mixed colony formation by control and infected FDCPmix cells. a Parental FDCPmix cells; b FDCPmix M3MuV-infected cells 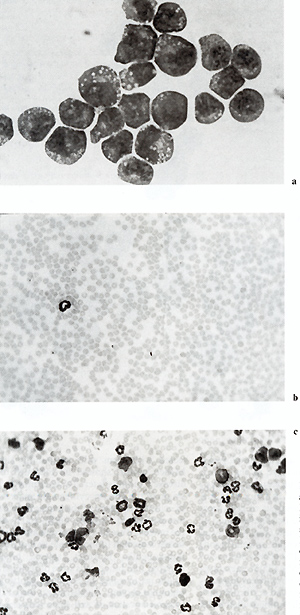 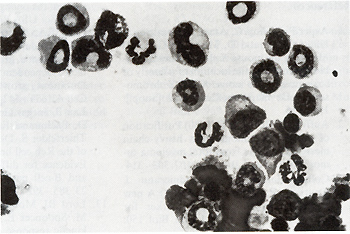
D. Discussion The present results show that infection of multipotent 11-3-dependent stem cells with a retroviral vector containing the 11-3 gene confer density-dependent autocrine stimulation of growth without blocking differentiation, but with a change of the balance between differentiation and proliferation in favor of proliferation. From these data we conclude that inappropriate expression of 11-3 may play an important role in the multistep pathogenesis of leukemia. When the 11-3 infected cells were injected into sublethally irradiated syngeneic mice, the animals developed a myeloproliferative disease. However, the precise role of the injected cells remains to be determined. Analysis of spleen and blood cells of the leukemic mice revealed that the proliferating cells were initially derived from the transplanted stem cells but were subsequently of recipient origin. Furthermore, the viral integration sites in cell lines recovered from leukemic animals showed different bands as compared with the original injected cells, indicating infection of host cells. Since FDCPmix cells contain an ecotropic helper virus (MoMuLV) [23], it could have packaged the defective MuV vector to produce an infectious virus which may then have transformed host cells. However, when high levels of the original MuV virus are injected into mice no myeloproliferative disease is observed [9]. In the latter case, this may reflect a difficulty in the ability of the injected virus to "target" to the host cells in the sites of active hematopoiesis. This may not be the case for the MuV-infected FDCPmix cells, which can clearly lodge in the spleen and bone marrow and may be acting as "carriers" for infectious viral particles, thus facilitating infection of host hematopoietic cells. Also, it has been reported that injection of recombinant 11-3 into normal mice leads to an increase in spleen weight and content of CFU -S, as well as to an increase in progenitor cells of the myeloid lineage [8, 13, 15]. Therefore, 11-3 production by the infected cells (both donor and host) may have contributed to the disease by stimulating stem and progenitor cells from the recipient mice. Thus, the disease is probably multifactorial. Nonetheless, we have clearly shown that endogenous inappropriate expression of a growth factor gene can have profound biological effects and may well be apart of the process leading to leukemic transformation.
1. Avner P, Bishop C, Amar L, Cambrou J, Hatat D, Arnaud D, Mattei M-G (1987) Mapping the mouse X chromosome possible symmetry in the location of a family of sequences on the mouse X and Y chromosomcs. Developmcnt-Camb 101 [Suppl]: 107-116 2. Auffray C, Rougeon F (1980) Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem 107: 303314 . Colbere-Garapin F, Horodniceanu J, Kourilsky P, Garapin A-C (1981) A new dominant hybrid selective marker for higher eukaryotic cells. J Mol BioI 150: 1-14 4. Feinberg AP, Vogelstein B (1984) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochcm 137: 266-267 5. Fialkow PJ, Singer JW (1985) Tracing developmcnt and cell lineages in human hemopoietic neoplasia. In: Weissman IL (ed) Leukemia. Dahlem Workshop. Springer, Berlin Heidelberg New York, pp 202- 222 6. Fialkow PJ, Singer JW, Adamson JW, Vaidya K, Dow LW, Ochs J, Moohr JW ( 1981) Acute nonlymphocytic leukemia: heterogeneity of stem cell origin. Blood 57:1068-1073 7. Greavcs MF, Delia D, Robinson J, Sutherland R, Newman R (1981) Exploitation of monoclonal antibodies: a "who's who" of haemopoietic malignancy. Blood Cells 7: 257-280 8. Kindler V, Thorens B, de Kossodo S, Allet B, Eliason JF (1986) Stimulation ofhematopoiesis in vivo by recombinant bacterial murine intcrleukin 3. Proc Natl Acad Sci USA 83:1001-1005 9. Laker C, Kluge N, Stocking C, Just U, Franz M-J, Ostertag W, Dexter M, Katsuno M, Spooncer E (1989) Leukemogenesis initiated by abcrrant multi-CSF cxpression is a multi-step process and differs in hcmatopoictic stem and precursor ccll lines. Mol Cell BioI (in press) 10. Laker C, Stocking C, Bergholz U, Hess N, DeLamarter JF, Ostertag W (1987) Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gcne and autonomous growth are distinct but inter-dependent steps in the oncogenic pathway. Proc Natl Acad Sci USA 84: 8458- 8462 11. Lang RA, Metcalf D, Gough N, Dunn AR, Gonda TJ (1985) Expression of a haemopoietic growth factor cDNA in a factor-dependent ccll line results in autonomous growth and tumorigenicity. Cell43:531-542 12. Lau B, Jaeger G, Thiel E, Rodt H, Huhn D, Pachmann K, Netzel B, Boening L, Thierfelder S, Doermer P (1979) Growth of the Reh cell line in diffusion chambers. Evidcnce for differentiation along the T and B-cell pathway. Scand J Haematol 23:385-392 13. Lord BJ, Molineux G, Testa NG, Kelly M, Spooncer E, Dexter TM (1986) The kinetic response of haemopoietic precursor cells, in vivo, to highly purified, recombinant interlcukin-3. Lymphokine Res 5:97104 14. McMastcr GK, Carmichacl GG (1977) Analysis of singlc- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci USA 74: 4835-4839 15. Metcalf D, Begley CG, Johnson GR, Nicola NA, Lopcz AF, Williamson DJ (1986) Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood 68:46-57 16. Miller AD, Buttimorc C (1986) Rcdcsign of retrovirus packaging cell lines to avoid rccombination leading to hclpcr virus production. Mol Cell Bioi 6:2895-2902 17. Rowley JD (1985) Significance of chromosome rearrangements in leukaemia and lymphoma. In: Weissman IL (ed) Leukemia. Dahlem Workshop. Springer, Bcrlin Heidelberg New York, pp 179-202 18. Robcrts RA, Spoonccr E, Parkinson EK, Lord BI, Allcn TD, Dexter TM (1987) Metabolically inactive 3T3 cells can substitute for marrow stromal cclls to promote the proliferation and development of multipotent haemopoietic stem cells. J Cell Physiol 132: 203- 214 19. Spooncer E, Bocttiger D, Dextcr TM (1985) Continuous in vitro generation of mulitpotential stem ccll clones from srcinfected culturcs. Nature 310:228-230 20. Spooncer E, Heyworth CM, Dunn A, Dcxter TM ( 1986) Self-renewal and differ entiation of intcrleukin-3-dependent multipotent stem cells are modulatcd by stro mal cells and serum factors. Differentiation 31: 111-118 21. Spooncer E, Lord BI, Dexter TM (1985) Defective ability to self-renew in vitro of highly purified primitive haematopoietic cells. Nature 316: 62-64 22. Stocking C, Kollek R, Bergholz U, Ostertag W (1985) Long terminal repeat sequences impart hematopoietic transformation properties to the myeloproliferative sarcoma virus. Proc Natl Acad Sci USA 82:5746-5750 23. Wyke JA, Stoker AW, Searle S, Spooncer E, Simmons P, Dexter TM (1986) Perturbed hemopoiesis and the generation of mulitpotential stem cell clones in src-infected bone marrow cultures is an indirect or transient effect of the oncogene. Mol Cell BioI 6:959-963 |