|
* Laboratory of Tumor Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Md., USA T -cell growth factor (TCGF), also called interleukin-2, supports proliferation oflectinor antigen-activated T cells. It was originally discovered in the conditioned media of phytohemagglutinin-stimulated peripheral blood lymphocyte (PEL) cultures [ 1,2]. It is also produced by some leukemic cell lines (e.g., Jurkat) after stimulation and, constitutively, by certain retrovirus-infected neoplastic T -cell lines [3]. TCGF produced by normal human PEL cultures has been purified to molecular homogeneity by biochemical means using a multistep procedure. First, the lymphocyte-conditioned media (Ly-CM) were concentrated 40-fold by diafiltration using the Millipore Pellicon Cassette system. The filter used was the polysulfate filter PTGC (10000 NMWL). Serum-containing media were further processed by anion-exchange chromatography: the concentrate was loaded onto a diethylaminoethyl-(DEAE)-sepharose column and eluted with an NaCI gradient in Tris buffer. TCGF activity of the collected fractions was determined in a [³H] thymidine incorporation assay using a cloned TCGF-dependent mouse-cell line (CTLL). When starting with serum-free media anion-exchange chromatography was unnecessary. In the next step Ly-CM concentrate or the active fractions of the DEAE-sepharose column, respectively, were adsorbed to controlled-pore glass (Electronucleonics). After overnight incubation in roller bottles the glass beads were packed into a column, washed with phosphate-buffered saline (PBS-Dulbecco) and Tris butler, and eluted with Tris buffer containing tetramethylammonium chloride. After extensive dialyzation against Tris buffer fractions were assayed for TCGF activity. Active fractions of the controlled-pore glass step were acidified with trifluoroacetic acid (TF A) and loaded onto a reverse-phase highperformance liquid chromatography (RPHPLC) column. The column was washed with 30% and 50% aqueous acetonitrile acidified withTFA; then it waseluted with 65% aqueous acetonitrile. To remove remaining impurities the eluate was diluted twofold with water and reloaded onto RP-HPLC. In the final step the column was washed with 40% aqueous acetonitrile and then developed with a gradient between 40% and 65% aqueous acetonitrile. The effluent was monitored by measuring the absorbance at 214 nm. TCGF eluted as a single peak at 60% aqueous acetonitrile. The degree of purification of the different steps and the recovery are shown in Table I. Molecular homogeneity of the purified TCGF was proved by determination of the NH2-terminal amino acid sequence by Edman degradation using a microprocedure [4]. Pure TCGF was able to support the long-term growth of human and murine T cells. Table I. Purification of TCGF from
lymphocyte-conditioned media 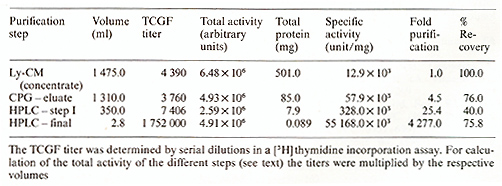
1. Morgan DA, Ruscetti FW, Gallo RC (1976) Science 193:1007-1008 2. Ruscetti FW, Morgan DA, Gallo RC (1977) J ImmunoI119:134-138 3. Gootenberg JE, Ruscetti FW, Gallo RC (1982) J Immunology 129: 1499-1505 4. Copeland TD, Grandgennett DP, Oroszlan S (1980) J Virol36: 115-119 |