|
A. Summary In the last 4 years we have established five long-term cultures from tumor material of Hodgkin's disease. The in vitro cells have malignant characteristics and represent the in vivo Hodgkin- and Sternberg-Reed-cells as shown by the identity of multiple properties. Common immunological, functional, and morphological assays did not characterize the in vitro cells as a known cell type of lymphoid, myeloid, or monocytoid tissue. The in vitro Hodgkin's disease cells are biologically active by producing factors involved in regulation and promotion of immunological response and granulopoiesis. The relevance of the findings for pathogenesis and clinical appearance of Hodgkin's disease is discussed.
Hodgkin's disease is still one of the most challenging entities in hematooncology. Of patients with this histopathological diagnosis, 60%- 70% can possibly be cured [3, II] and 30%-40% do not respond with complete remission upon first treatment; of this group 15%-20% will achieve remission upon secondary treatment, but 15%-20% will die within 4-18 months in spite of intensive treatment strategies (Fig. I). Secondary neoplasias are the most hazardous consequence of intensive combined treatment modalities (radiochemotherapy) at the moment and amount to 5%-10% of all initially diagnosed Hodgkin's disease patients [ I, 2, 16]. The incidence of acute myeloid or myelomonocytoid leukemia is 130 times higher in Hodgkin's disease patients than in normal individuals [9]. Attempts to investigate the nature of the pathognomonic Hodgkin- (H) and Sternberg-Reed (SR)-cells have been hampered by the fact that these cells constitute only a small minority in the primary biopsy and seem to be utterly growth restricted in the hitherto available culture systems [6]. Since 1978 we have established five cell lines from tumor-involved specimens derived from HD patients [5, 6, 12]. All histologies were confirmed by four independent hematopathologists.
All five lines were grown from HD specimens of four patients with
nodular sclerosing-type histologies, clinical stage IVB. The patients
had been submitted to intensive combination chemoradiotherapy. The
sources for the culture material were pleural effusions in three
cases and bone marrow and peripheral blood in one case. Two identical
cultures were established from the two different sources of this
particular patient. Four cell lines continously proliferate in vitro,
while on line stopped growing for unknown reasons after 7 months
in culture (L 439). 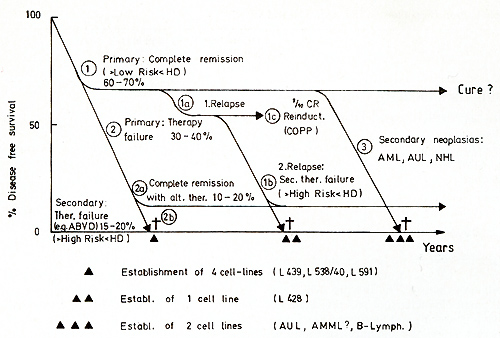
The cell lines share an identical (L 428) or partially identical phenotype (L 538/540, L 591) with in vivo H- and SR-cells and represent a cell type previously unknown according to the methods used for cellular discrimination [15]. The neoplastic nature of the five HO tumor cell lines is indicated by aneuploidy, except in one line (L 591), and multiple structural and numerical chromosome abnormalities associated with a monoclonal pattern of multiple marker chromosomes. A comparison of the characteristic features of the in vitro cultured HO cells with H- and SR-cells from freshly obtained biopsies is shown in Table I. All cultured cells lacked surface- or cytoplasmic-Igs. IgG present in fresh biopsy H- and SRcells was not found in vitro. Ia-like antigens, receptors for human T cells, acid phosphatase, and acid naphthol acetate esterase were present in all cultured lines. EBV specific receptors were found in two out of two tested lines, EBV genomes and EBV -induced antigens, however, only in one line (L 591). All HO cell lines as well as fresh biopsy H- and SR-cells are devoid of HTLA receptors for C3b, C3d, IgG-Fc, mouse-E, or sheep-E and of lysozyme, peroxidase, and chloracetate esterase (Table I). The identity of the in vivo and in vitro H- and SR-cells was shown by congruent morphological, functional, and immunological markers. The strongest proof for the derivation of the cultured cells from Hand SR-cells in vivo was the demonstration of cross-reacting surface and cytoplasmatic constituents on the in vivo and in vitro cells by means of absorbed polyclonal (rabbit anti L 428 cells) (Table I) and mouse monoclonal (anti L 428 cells) antibodies (Ki I, Ki 24, Ki 27) (Table 2). Furthermore, monoclonal antibodies directed against .granulopoietic cell determinants (3C4, TU 9) were also present on biopsy HO cells and the cell lines L 428 KS and L 540, but were absent on the L 591 cells. In an attempt to determine the origin and nature of the cultured H- and SRcells a multitude of monoclonal antibodies directed against human lymphoid and hematopoietic differentiation markers were tested against these cells. Table 3 summarizes the results by showing the most commonly accepted markers as specific attributes of the different cell types. As demonstrated in this table, the reactive pattern of the cell line L 428 and partially of the cell line L 540 was identical Table 1. Properties of in vivo and in
vitro H- and SR-cells 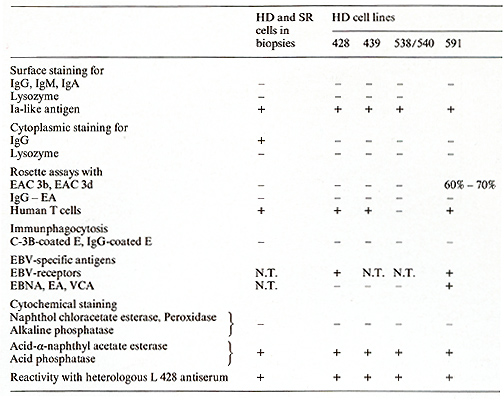
Table 2. Reactivity of HO and SR cells
in biopsies and in vitro (lines) 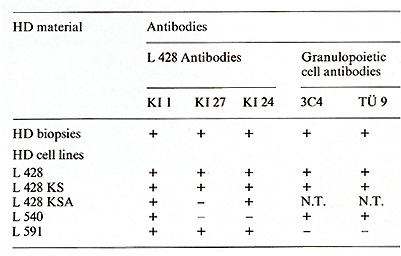 Table 3. Differential characteristics of HD and SR cells and HD cell lines 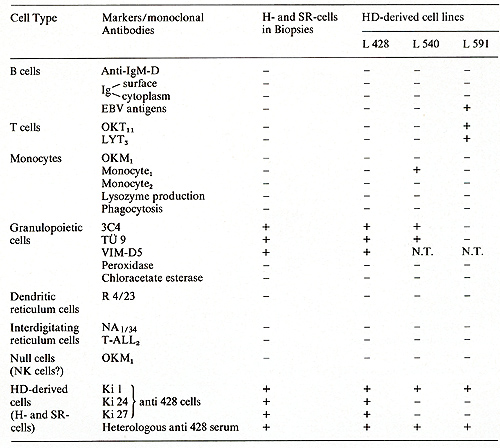 Table 4. Biological activities of HD line supernatants 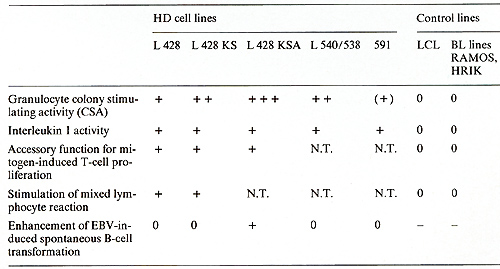
E. Conclusions 1. L 428 respresents in vitro the in vivo H- and SR-cell population.
The other reported lines exhibit most but not all markers of in
vivo H- and SR-cells. The non-LCL character of L 591 is still the
subject of discussion. 2. The HO cells (in vivo and in vitro) represent
no known cell type or any cell class so far identified by common
immunological, functional, and morphological tests. 3. The in vitro
HO cells exhibit biological activities regulating and/ or promoting
immune response and granulopoiesis. F. Hypothetic Pathogenesis of Hodgkin's Disease Figure 2 summarizes all the data obtained on the described HO-derived
cell lines in an attempt to propose a hypothesis for some of the
pathogenetic mechanisms involved in Hodgkin's disease: The origin
of H- and SR-cells in unknown. It is possible that the Ki 1 antibody
not only recognizes H-SR-specific determinants, but also depicts
a previously 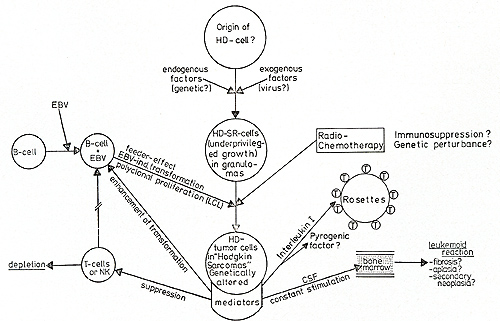
1. Borum K (1980) Increasing frequency of acute myeloid leukemia complicating Hodgkin's disease: A review. Cancer 46: 1247-1252 2. Coltman CA, Dixon DO (1982) Second malignancies complicating Hodgkin's disease: A southwest oncology group 10-year follow up. Cancer Treat Rep 66: 1023-1034 3. De Vita VT, Lewis BJ, Rosenzweig Met al. (1978) The chemotherapy of Hodgkin's disease. Cancer 42: 979-990 4. Diehl V, Johannson B (1977) Establishment of peripherallymphoid cell cultures from patients with Hodgkin's disease (HD) depending on Epstein-Barr virus (EBV) reactivity and cellular immunity. Blut 34: 227 -236 5. Diehl V, Kirchner HH, Schaadt Met al. (1981) Hodgkin's disease: Establishment and characterization of four in vitro cell lines. J Cancer Res Clin Oncoll01 : 111-124 6. Diehl V, Kirchner HH, Burrichter H, Stein H, Fonatsch C, Gerdes J, Schaadt M, Heit W, Uchanska-Ziegler B, Ziegler A, Heinz F, Sueno K (1982) Characteristics of Hodgkin's disease-derived cell lines. Cancer Treat Rep 66:615-632 7. Fisher RI, Bostick-Bruton F, Diehl V (to be published) Neoplastic cells obtained from Hodgkin's disease function as accessory cells for mitogen-induced, human T -cell proliferative responses 8. Fisher RI, Bostick-Bruton F, Sander DN , Diehl V (to be published) Neoplastic cells obtained from Hodgkin's disease are potent stimulators of human primary mixed lymphocytic cultures 9. Glicksman HS, Pajak TF, Gottlieb A et al. (1982) Second malignant neoplasms in patients successfully treated for Hodgin's disease: A cancer and leukemia group B study. Cancer Treat Rep 66: 1035-1044 10. Kaplan HS (1981) Hodgkin's disease: Biol ogy, treatment, prognosis. Blood 57:813 11. Longo Dl, Young RC, De Vita VT (1982) The chemotherapy for Hodgkin's disease: The remaining challenges. Cancer Treat Rep 66:925-936 12. Schaadt M, Diehl V, Stein H et al. (1980) Two neoplastic cell-1ines with unique features derived from Hodgkin's disease. Int J Cancer 26: 723- 731 13. Schwab U, Stein H, Gerdes J, lemke H, Kirchner HH, Schaadt M, Diehl V (1982) Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature 299:65 14. Stein H, Gerdes J, Kirchner HH et al. (1981) Hodgkin's disease. Imm unohistological analysis of Hodgkin- and Sternberg-Reed cells. J Cancer Res C1in Onco1101: 125-134 15. Stein H, Gerdes J, Schwab U, lemke H, Mason DY, Ziegler A, Schienle W, Diehl V ( 1982) Identification of Hodgkin- and Sternberg-Reed cells as a unique cell type derived from a newly detected cell population. Int J Cancer 30: 445-459 16. Valagussa P, Santaro A, Kenda R et al. (1980) Second malignancies in Hodgkin's disease: A complication of certain forms of treatment. Br Med J 280:216-219 |