|
A. Introduction
Human T cell leukemia virus (HTL V) is the name by which we have
designated a family of related retroviruses from humans, HTLV type
I (HTLV-I) is the name we gave the first human retrovirus isolate,
HTLV-I is endemic at low rates in different parts of the world,
including southern Japan, the Caribbean, South and Central America,
the southeastern United States, and especially in Africa, Seroepidemiologic
studies show that HTL V-I is the primary etiologic agent of an aggressive
form of adult T cell leukemia/lymphoma (ATLL), Infection with HTLV-I
in vivo occurs preferentially with OKT4+ T cells and results in
immortalization of the infected cells as well as abrogation of various
immune functions of the infected cells, in keeping with its role
in the etiology of ATLL, A second related but distinct virus, HTL
V type II ( HTL V-II ), was identified by us in collaboration with
D, Golde and colleagues after type I, in material from a patient
with hairy cell leukemia, HTLV-II shares many features with HTLV-I,
including in vitro transforming activity, but it has been isolated
only rarely and has not yet been associated with any disease, A
third virus, HTL V type III (HTL V-III), has been isolated many
times from individuals who have acquired immunodeficiency syndrome
(AlDS) or are at risk for this disease, HTLV-lII shares some antigenic
cross-reactivity with land Il, as well as some general features,
including an OKT4+ T cell tropism, The virus is more highly infectious
than lor II, however, and has so far shown only cytopathic and not
immortalizing effects, Seroepidemiologic data show that HTLV-lll
is the cause of AIDS.
B. HTL V -I and Adult T CeII Leukemia/Lymphoma
The first human retrovirus isolates were obtained from malignant
T cell lines established with the use of T cell growth factor (TCGF),
a protein present in the media of peripheral blood cells stimulated
with phytohemagglutinin [ l, 27, 40], The T cell lines were established
from black patients in the United States with what were diagnosed
as unusually aggressive variants of cutaneous T cell lymphoma [28,
29, 35], The virus, which we called HTLV-I, has typical retrovirus
morphology ( Fig, l) and, like other retroviruses, contains both
a reverse transcriptase and high-molecularweight polyadenylated
genomic RNA, HTLV-l was shown to be unique by the criteria of protein
serology [l4, 37, 38] and nucleic acid hybridization [35], and to
be exogenous to man [35], Transmission is horizontal and does not
occur genetically [9,54],
The isolation of HTL V-I made it possible to make antibodies to
the viral proteins, These antibodies were then used to test serum
sam pies for the presence of HTL V -I. Most persons in the Unitcd
States were negative for this virus, including patients with many
types of leukemia and lymphoma, HTLV-I was detected in a small fraction
of persons from the United States with cutaneous T cell leukemia
or lymphoma, most of whom were blacks in the southeastern United
States or of Caribbean origin [4, 30]. Even most of these patients
were negative.
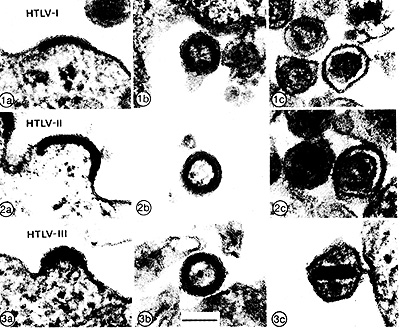
Fig. 1. Electron microscopy of HTLV-I,
II, and III. Shown are budding (panels a), immature (panels b),
and maturc (panels c) virions of the three types of HTL V. The bar
in 3 b equals IOOnm
Two regions of the world were identificd, however, in which thcre
were endemic discases which clinically resemblcd those from which
the first two isolates ofHTLV-I werc obtained, These regions were
the Caribbean [5] and southwestern Japan [51]. The disease in the
Caribbean was called lymphosarcoma cell leukemia, and that in Japan
was called adult T cell leukemia; both were found to be closely
associated with the presence of HTLV-I by seroepidemiology [3, 13,
39]. Both diseases are now regarded as the same clinical entity,
and are collectively called adult T cell leukemia/lymphoma (ATLL).
Similar results have been reported by investigators in Japan, who
also isolated retroviruses from A TLL cell lines [25, 54]. These
retroviruses are now known to be isolates of HL TV-I [52]. Sporadic
occurrences of both HTLV-I and ATLL have been noted in many other
areas of the world [ 10], and most recently parts of Africa have
also been shown to be endemic [43].
As is true for the naturally occurring ani
mal leukemia viruses, only a small fraction of HRV-I-infected people
develop leukemia [50]. It thus appears as though other factors,
such as the host immune response, age at exposure, virus dose, or
route of infection, may be important factors in determining the
end result of infection.
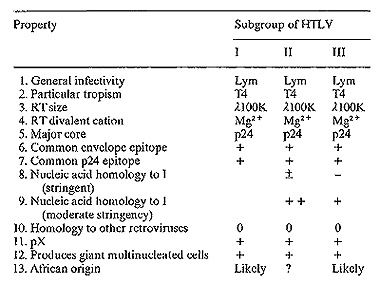
Table I. Relatedness of HTLV-I, II, and
III
C. In Vitro Biological Effects of HTL V -I
HTLV-I was first shown by Miyoshi et a]. to transform T cells [26],
but the target cells were not shown to be initially free of virus.
Subsequently, transformation was achieved using target T cells shown
to be HTL V -I negative [31,32].
HTL V- I is tropic for T cells of the O KT4 + phenotype both in
vivo [9] and in vitro [19, 31, 32]. Transmission is achieved easily
by co-cultivation with killed virus-producing cells, but only with
difficulty when cell-free virus is used. The infected cells take
on many of the properties of transformed ATLL cells, including altered
morphology, increased growth rate, the tendency to grow in clumps,
reduced dependence on TCGF, expression of high levels of the TCGF
receptor and HLA-Dr antigens on the cell surface, and (usually)
immortalization in culture [22,23, 31, 32]. In vitro transformation
by HTL V- I seems to be m uch more rapid and efficient than leukemogenesis
in vitro.
Infection with HTLV-I of functional T cells results in the loss
of some or all of their immune functions. For example, a T cell
line which was cytotoxic for autologous tumor cells was established
from one (rare) long-term survivor of ATLL [22]. These cells were
themselves infectable with HTLV-I, and one clone ofinfected cells
was shown to have lost the ability to kill its target cells. Instead,
the cell would stop dividing and die when presented with the target
[23]. Various other functional losses after infection with HTL V-I
have been reported in addition [24, 34]. HTL V-I also infects bone
marrow cells in vitro, giving rise to T cell lines of different
phenotypes, including OKT4+T8-, OKT4-+, and OKT4-8-.
D. HTLV-II
HTL V-II was originally isolated from a patient with hairy cell
leukemia [16]. Although it shares antigenic determinants of the
major gag protein, p24, and the envelope proteins [16, 18] of HTLV-I,
it is readily distinguishable by both protein serology [ 17] and
nucleic acid hybridization [36]. It has many common biochemical
properties with HTLV-I (see Table I), including the ability to transform
T cells in vitro and to mediate a loss ofimmune functions [34].
It has been isolatcd only twice, and in spite of its biological
activity in vitro it is not clear at this time with what disease,
ifany, it is associated.
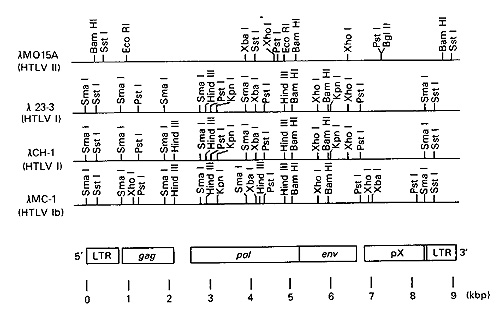
Fig.2. Genomes and rcstriction maps of
HTL V-I and II. lamda MO15A is an example of HTLV-II, lamda 23-3
and lamda CH-1 are examples of HTLV-l, and lamda MC1 is HTLV-Ib.
Genomic regions corresponding to L TR, gag, pol, env, and pX are
drawn to scale according to the publishcd nucleotide sequence of
an HTL V-I isolate. Two Bg1ll sites in thc 5' end of lamda MO 15A
are not shown
E. Genomes of HTLV-I and HTLV-II
The genome of HTL V -I has been completely sequenced [45]. HTLV-I
contains two large terminal repeat (L TR) sequences, in common with
other retroviruses, which contain transcriptional control signals.
There are fairly typical gag, pol, and env genes, although the gag
gene seems to code for three proteins rather than four. In addition,
there is an extensive stretch of DNA 3' to the env gene, which contains
several potential open reading frames capable of coding for proteins.
This is called the pX region, and does not seem to be necessary
for viral replication. It may be important in cell transformation,
as discussed below, but it is not a cell-derived onc gene, since
it has no homology with host cell DNA, The structure of the HTLV-I
genome is shown in Fig. 2.
The HTLV-II genome also contains a pX region, and has the same gene
order as HTL V-I [46]. Heteroduplex analyses using relaxed hybridization
conditions indicate that the two viruses are at least distantly
related over the length of their genomes. The 3' portion of pX region
seems to be the most closely conserved part of the genome. The HTLV-II
pX has been recently sequenced [23], and the 3' part of this sequence
has a large open reading frame which has the coding potential for
a protein of at least 38 kilodaltons. The close homology with the
analogous region of the HTLV-I genome suggests that the product
for which these regions code is important for the biological activity
of these viruses.
The env gene sequence of HTLV-II has also been recently reported
[47], and it also shows significant homology with the HTLV-I env
gene, except for the extreme 3' and 5' termini, The L TRs of the
two viruses are markedly different over most of their length [49],
but small regions near the RNA cap site, the primer binding site,
and a 21base pair sequence present at four copies in the HTL V -II
L TR and three copies in the HTL V-I L TR are highly homologous.
These last sequences could represent RNA transcriptional enhancers.
How do HTL V-I and II transform T cells? One puzzling aspect of
the molecular biology of HTLV-I and II is that although transformation
of infected cells is rapid, the viral genome does not contain atypical
(i,e" cell-derived) onc gene. Moreover,leukemogenesis appears
to be relatively inefficient and to involve a long latent period,
as with the chronic animal leukemia viruses.
A second puzzling feature of transformation is that the proviral
integration site in fresh leukemic blood cells, leukemic cell lines,
and cord blood T cell lines transformed in vitro is nearly always
mono- or oligoclonal [23, 53-55], suggesting that only a few of
the infected cells become transformed. There does not, however,
seem to be a preferential integration site common to different leukemic
patients or cell lines [53, 55], suggesting that a specific integration
site is not required for transformation, and that the viral genome
itself contains all the necessary information.
What is the reason for these apparent paradoxes? It has been shown
that the activities of the HTLV-I and II RNA polymerase promoters
are ,strongly intlucnccd by the cell type in which they are present
[6, 48], and are far more active in T cells than in other cells.
Activity is higher in cells already infected with HTL V than in
uninfected cells. This has been interpreted as indicative of the
presence of a trans-acting factor present in HTL V-infected cells,
which strongly activates the HTL V promoter. Sodroski et al. [48]
suggest that this factor may in fact be the pX product. If this
were the case, and if it had the ability to af-fect the promoters
of cellular genes necessary for T cell function and growth, it could
help to explain both rapid transformation by HTLV without the requirement
for a specific integration site and a cytopathic or dysfunctional
effect on infected T cells. It does not explain, however, the monoclonality
of transformed cell populations with respect to the viral integration
site.
F. HTL V -III and AIDS
Acquired imm unodeflciency syndrome
(AIDS) is a recently recognized, generally fatal disease involving
helper T cell depletion and multiple opportunistic infections and/or
malignancies. It is prevalent among certain high-risk groups, including
promiscuous homosexuals, intravenous drug abusers, hemophiliacs,
Haitians, and in fants born to members of high-risk groups. Because
epidemiologic data suggested involvement of a transmissible agent
and because of the involvement of OKT4 + T cells in the disease,
it seemed possible that an HTL V-Iike retrovirus might be involved.
Essex et al. reported the presence of an antibody present in a large
percentage of AIDS victims and high-risk populations which reacted
against a cell surface protein ofHTLV-I-infected cells [7,8].
Recently, we reported on a cell line permissive for the growth of
a retrovirus from AIDS and pre-AIDS patients [33]. More than 90
isolates from this group of viruses have been obtained [ 1]; P.
Markham et al., in preparation]. Based on morphology, biochemical
properties of reverse transcri ptase [33], antigenic determinants
of env and gag proteins [44], and demonstration of distant but significant
nucleic acid homology in the gag-pol region, this new virus is distantly
related to HTLV-I and II, and has been designated HTLV-III. A more
detailed characterization of HTL V-III is given by Wong-Staal et
al. (this volume).
The distant relatedness of these viruses suggests that the antibody
activity described by Essex and his colleagues retlected crossreactivity
of HTLV-I antigen with antibodies to HTLV-III. We have isolated
HTL V-III from a majority of pre-AIDS patients and a large number
or actual AIDS patients [1]], but isolation from the normal population
is rare. Almost all AIDS and pre-AIDS patients have antibodies to
HTL V-III [42]. A typical Western blot is shown in Fig. 3. The major
reactivity is against a 41K protein, which is probably the env antigen
of HTLV-III. The most recent data show that the prevalence of such
antibodies in these patients is virtually 100% [41]. The association
is so striking as to overwhelmingly suggest that this virus is the
cause of AIDS. Recent evidence indicates that the virus called ALV
or IDAV, detected previously by Barre-Sinoussi et al. [2], is a
member or the same HTLV subgroup.
These accumulated data indicate that there is a group of related
human retroviruses with disparate effects on the same target cell,
the OKT4+ T cell.
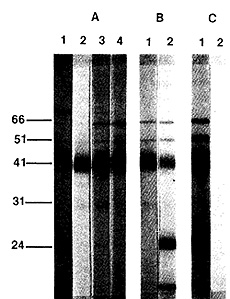
Fig.3. Analysis of sera for antibodies
to HTL VIII by Western blot. A. Sera from AIDS patients; B sera
from lymphadenopathy patients; C a positive and a negative serum
from homosexual subjects. Numbers refer to the molecular weight
in kilodaltons
It will be interesting to see whether there are other similar viruses
that have yet to be discovered. The identification of the present
members of this group gives us opportunities to study T cell biology,
as well as the potential to intervene in certain now fatal (and
at least in the case of AIDS, increasingly prevalent) T cell diseases.
References
1. Arya SK, Gallo RC, Hahn BH, Shaw GM, Popovic M, Salahuddin SZ,
Wong-Staal F (1984) Homology of genome of AIDS-associatcd virus
(HTL V-III) with genomes of human T-cellleukcmia viruses (HTLV-I
and HTLV-II). Science 225.927-930
2. Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S,
Gruest J, Dauguet C, Axler-Blin C, Veinet-Brun F, Rouzioux C, Rosenbaum
W, Montagnier L (1983) Isolation of a T-Iymphotropic retrovirus
from a patient at risk for acquired immune deficiency syndrome (AIDS).
Science 220: 868-870
3. Blattner W A, Kalyanaraman VS, RobertGuroff M, Lister T A, Galton
DAG, Sarin PS, Crawford MH, Catovsky D, Greaves M, Gallo RC ( 1982)
The human type-C retrovirus, HTL V, in Blacks from the Caribbean
region, and relationship to adult T -cell leukemia/ lymphoma. Int
J Cancer 30: 257-264
4. Blayney DW, Blattner WA, Robcrt-Guroff M, Jaffe ES, Fisher RI,
Bunn PA Jr, Pat ton MG, Rarick HR, Gallo RC (1983) The human T-cellleukemia-lymphoma
virus in the southeastern United States. JAMA 250. 1048 1052
5. Catovsky D, Greaves MF, Rose M, Galton DAG, Goolden A WG, McCluskey
DR, White JM, Lampert I, Bourikas G, Ireland R, Brownell AI, Bridges
JM, Blattner WA, Gal10 RC (1982) Adult T-cell lymphoma-leukaemia
in blacks from the West Indics. Lancet I.639-643
6. Chen ISY, McLaughlin J, Goldc DW ( 1980) Long tcrminal repeats
of human T-cell leukemia virus II genomc determine target cell specificity.
Nature 309 276-280
7. Essex M, McLane MF, Lee TH, Falk L, Howe CWS, Mullins JI, Cabradilla
C, Francis DP (1983a) Antibodies to cell mcmbrane antigcns associated
with human T-cell leukemia virus in patients with AIDS. Science
220.859-862
8. Esscx M, McLane MF, Lee TH, Tachibana N, Mullins JI, Kreiss J,
Kasper CK, Poon M-C, Landay A, Stein SF, Francis DP, Cabradilla
C, Lawrcnce DN, Evatt BL (1983b) Antibodies to human T-cell leukemia
virus membrane antigens (HTLVMA) in hemophiliacs. Science 221.1061
-1063
9. Gallo RC, Mann D, Broder S, Ruscetti FW, Maeda M, Kalyanaraman
VS, Robert-Guroff M, Reitz MS (1982) Human T-celllcukcmialymphoma
virus (HTLV) is in T- but not B-Iymphocytes from a patient with
cutaneous T -cell lymphoma. Proc Natl Acad Sci USA79.4680-4684
10. Gallo RC, Kalyanaraman VS, Sarngadharan MG, Sliski A, Vonderheid
EC, Maeda M, Nakao Y, Yamada K, Ito Y, Gutensohn N, Murphy S, Bunn
PA Jr, Catovsky D, Greaves MF, Blayney DW, Blattner W, Jarrett WFH,
zur Hausen H, Seligmann M, Brouet JC, Haynes BF, Jegasothy BV, Jaffe
E, Cossman J, Broder S, Fisher RI, Golde DW, Robert-Guroff M (1983)
Association of the human type C retrovirus with a subset of adult
T -cell cancers. Cancer Res 43 3892 -3899
II. Gallo RC, Salahuddin SZ, Popovic M, Shearer GM, Kaplan M, Hayncs
BF', Palkcr TJ, Redfield R, Oleske J, Satai B, White G,
Foster P, Markham PD (1984) Frequent detection and isolation of
cytopathic retroviruses (HTL V-III) from patients with AIDS and
at risk for AIDS. Science 224: 500-503
12. Hahn BH, Shaw GM, Arya SK, Popovic M, Gallo RC, Wong-Staal F
(submitted) Molecular cloning and characterization of the virus
associated with AIDS (HTLV-III)
13. Haseltine WA, Sodroski J, Patrarca R, Briggs D, Perkins D, Wong-Staal
F ( 1984) Structure of the 3' terminal region of type II human T
-lymphotropic virus. Evidence for a new coding region. Science 225:419-421
14. Kalyanaraman VS, Sarngadharan MG,
Poiesz BJ, Ruscetti FW, Gallo RC (1981) Immunological properties
of a type C retrovirus isolated from cultured human T-Iymphoma cells
and comparison to other mammalian retroviruses. J ViroI38:906-915
15. Kalyanaraman VS, Sarngadharan MG, Nakao Y, Ito Y, Aoki T, Gallo
RC (1982a) Natural antibodies to the structural core protein (p24)
of the human T -cell leukemia (lymphoma) retrovirus found in sera
of leukemia patients in Japan. Proc Natl Acad Sci USA 79.1653-1657
16. Kalyanaraman VS, Sarngadharan MG, Robert-Guroff M, Miyoshi I,
Blayney D, Golde D, Gallo RC (1982b) Anew subtype of human T -cell
leukemia virus (HTL V-II) associated with a T -cell variant of hairy
cell leukemia. Science 218.571-573
17. Kalyanaraman VS, Jarvis-Morar M, Sarngad ha ran MG, Gallo RC
(1984) Immunological characterization of the low molecular weight
gag gene proteins pl9 and pl5 ofhuman T -cell leukemia-Iymphoma
virus (HTLV) and demonstration of human natural antibodies to them.
Virology 132:61-70
18. Lee TH, Coligan JE, McLane MF, Sodroski JG, Popovic M, Wong-Staal
F, Gallo RC, Haseltine W, Essex M (in press) Serologic cross-reactivity
between envelope gene products of type I and type II human T -cell
leukemia virus Proc Natl Acad Sci USA
19. Mann DL, Popovic M, Murray C, Neuland C, Strong DM, Sarin P,
Gallo RC, Blattner WA (1983a) Cell surface antigen expression of
newborn cord blood lymphocytes infected with HTLV.J ImmunoI 131.2021-2024
20. Mann DL, Popovic M, Sarin PS, Murray C', Reitz MS, Strong DM,
Haynes BF, Gallo RC, Blattner WA ( 1983 b) Cell lines producing
human T -cell lymphoma virus show altered HLA expression Nature
305:58-60
21. Markham PD, Salahuddin SZ, Macchi B, Robert-Guroff M, Gallo
RC ( 1984) Transformation of different phenotypic types of
human bone marrow T-Iymphocytes by HTLVI. Int J Cancer 33. 13-17
22. Mitsuya H, Matis LA, Megson M, Bunn PA, Murray C, Mann DL, Gallo
RC, Broder S ( 1983) Generation of an HLA-restricted cytotoxic T
-cell line reactive against cultured tumor cells from a patient
infected with human T-cellleukemia/lymphoma virus. J Exp Med 158:994-999
23. Mitsuya H, Guo H-G, Megson M, Trainor CD, Reitz MS, Broder S
( 1984) Transformation and cytopathic effect in an immune T-cell
clone infected by human T-cell leukemia-Iymphoma virus (HTL V).
Science 223.1293-1295
24. Mitsuya H, Quo H-G, Cossman J, Megson M, Reitz M, Broder S (1984)
Functional properties of antigen-specific T -cells infected by human
T-cellleukemia/lymphoma virus (HTLV-I). Science 225: 1484-1486
25. Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi
Y, Nagato K, Hinuma Y (198Ia) Type C virus particles in a cord T
-cell line derived by co-cultivating normal human cord blood leukocytes
and human leukemic T-cells. Nature 294.770 -771
26. Miyoshi T, Yoshimoto S, Kubonishi I, Tagushi H, Shiraishi Y,
Ohtsuki Y, Akagi T (1981 b) Transformation of normal human cord
lymphocytes by co-cultivation with a lethally irradiated human T-cellline
carrying type C virus particles. Gann 71.155-156
27. Morgan DA, Ruscetti FW, Gallo RC (1976) Selective in vitro growth
of T-Iymphocytes from normal human bone marrow.
Science 193. 1007-1008
28. Poiesz BJ, Ruscetti FW, Gazdar AF. Bunn PA, Minna JD, Gallo
RC (1980) Detection and isolation of type C retrovirus particles
from fresh and cultured lymphocytes ofa patient with cutaneous T
-cell lym phoma. Proc Natl Acad Sci USA77:7415-7419
29. Poiesz BJ, Ruscetti FW. Reitz MS, Kalyanaraman VS, Gallo RC
( 1981) Isolation of anew type-C retrovirus (HTL V) in primary uncultured
cells of a patient with Sezary T-cellleukemia. Nature 294:268-271
30. Posner LE, Robert-Guroff M, Kal
yanaraman VS, Poiesz BJ, Ruscetti FW, Fossieck B, Bunn PA Jr, Minna
JD, GJallo RC (1981) Natural antibodies to the human T cell lymphoma
virus in patients with cutaneous T cell lymphomas J Exp Med 154.
333-346
31. Popovic M, Lange- Wantzin G, Sarin PS, Mann D, Gallo RC (1983a)
Transformation of human umbilical cord blood T cells by human T-cell
leukemia/lymphoma virus. Proc Natl Acad Sci USA 80:5402-5406
32. Popovic M. Sarin PS. Robert-Guroff M. Kalyanaraman VS. Mann
D. Minowada J. Gallo RC (1983b) Isolation and transmission ofhuman
retrovirus (human T-cellleukemia virus). Science 219:856-859
33. Popovic M. Sarngadharan MG. Read E. Gallo RC ( 1984) Detcction,
isolation. and continuous production of cytopathic retroviruses
(HTlV-III) from patients with AIDS and pre-AlDS. Science 224:497-500
34. Popovic M, Flomenberg N. Volkman Dl, Mann D. Fauci AS, Dupont
B. Gallo RC (1984) Alteration in T-cell functions by infection with
HTlV-I or HTlV-Iis Science 226:459-462
35. Reitz MS. Poiesz BJ. Ruscetti FW. Gallo RC ( 1981 ) Characterization
and distribution of nucleic acid seq uences of a novel type C retrovirus
isolated from neoplastic human T Iymphocytess Proc Natl Acad Sci
USA 78. 1887-1891
36. Reitz MS Jr, Popovic M. Haynes BF. Clark SC. Gallo RC ( 1983)
Relatedness by nucleic acid hybridization of new isolates of human
T -cell leukemia-Iymphoma virus (HTl V) and demonstration of provirus
in uncultured leukemic blood cells. Virology 126.688-692
37. Rho HM. Poiesz BJ. Ruscetti FW. Gallo RC ( 1981) Characterization
of the reverse transcriptase from a new retrovirus (HTl V) produced
by a human cutaneous T-cell lymphoma cell line. Virology 112.355-360
38. Robert-Guroff M. Ruscetti FW. Posner lE. Poiesz BJ, Gallo RC
(1981) Detection of the human T -cell lymphoma virus p 19 in cells
of some patients with cutaneous T-cell lymphoma and leukemia using
a monoclonal antibody. J Exp Med 154: 1957-1964
39. Robert-Guroff M. Nakao Y, Notake K, Ito Y, Sliski A. Gallo RC
(1982) Natural antibodies to human retrovirus HTl V in a cluster
of Japanese patients with adult T cellleukemia. Science 215:975-978
40. Ruscetti FW, Morgan DA, Gallo RC ( 1977) Functional and morphological
characterization of human T -cells continuously grown invitro.J
ImmunoI I19.131-138
41. Safai B, Sarngadharan MG. Groopman J. Arnett K. Popovic M. Sliski
A. Schupbach J. Gallo RC ( 1984) Seroepidemiological studies of
human T -lymphotropic retrovirus type III in acquired immunodeficiency
syndrome. lancet I: 1438-1440
42. Sarngadharan MG, Popovic M, Bruch l. Schupbach J. Gallo RC (1984)
Antibodies reactive with human T-Iymphotropic retroviruses (HTl
V-III) in the serum of patients with AIDS. Science 224.506-508
43. Saxinger WC. Blattner WA. levine PH, Clark J, Biggar R. Hoh
M, Moghissi J. Jacobs P. Wilson l. Jacobson P, Crookes R, Strong
M. Ansari AA, Dean AG. Nkrumah F.H, Mouvali N. Gallo RC (1984) Human
T-cellleukemia virus (HTl V-I) antibodies in Africa. Science 225.
1473-1476
44. Schupbach J. Popovic M. Gilden RV, Gonda MA. Sarngadharan MG,
Gallo RC (1984) Serological analysis of a su bgroup of human T-Iymphotropic
retroviruscs (HTlV-IlI) as5ociated with AIDS. Science 224.503-504
45. Seiki M, Hat tori S. Hirayama Y. Yoshida M (1983) Human adult
T-cell leukemia virus: complete nucleotide sequence of the provirus
genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA 80.3618
-3622
46. Shaw GM, Gonda MA, Flickinger GH, Hahn BH, Gallo RC, Wong-Staal
F (1984) The genomes of evolutionarily divergent members of the
human T -cell leukemia virus family (HTlV-I and HTlV-II) are highly
conserved, especially in pX. Proc Natl Acad Sci USA 81: 4544-4548
47. Sodroski JG, Patarca R, Perkins D. Briggs D, lee TH, Essex M,
Coligan J. Wong-Staal F, Gallo RC. Haseltine WA (1984a) Sequence
of the envelope glycoprotein gene of type II human T Iymphotropic
virus. Science 225. 421-424
48. Sodroski JG. Rosen CA, Haseltine WA ( 1984 b) Trans-acting transcriptional
activation of the long terminal repeat of human T -lymphotropic
viruses in infected cells. Science225.381-385
49. Sodroski JG, Trus M. Perkins D. Patarca R. Wong-Staal F, Gelmann
E, Gallo R. Haseltine W A (in pres) Repetitive structure in the
long terminal repeat element of type II human T cell leukemia virus.
Proc Natl Acad Sci USA
50. Tajima K, Tominaga S. Suchi T, Kawagoe T, Komoda H, Hinuma Y.
Oda T, Fujita K (in press) Epidemiological analysis on distribution
of antibody to adult T-cellleukemiavirus-associated antigen (A TLA):
possible horizontal transmission of adult T-cell leukemia virus.
Gann
51. Takatsuki K, Uchiyama J, Sagawa K. Yodoi J (1977) Adult T-cellleukemia
in Japan. In. Seno S, Takaku F, Irino S (eds) Topics in hematology.
Excerpta Medica. Amsterdam, pp 73- 77
52. Watanabe T. Seiki M. Yoshida M (1984) HTlV type I (U.S. isolate)
and ATlV (Japanese isolate) are the same species of human retrovirus.
Virology 133: 238-241
53. Wong-Staal F, Hahn B, Manzari V, Colombini S. Franchini G. Gelmann
EP, Gallo RC (1983) A survey of human leukaemia for sequences of
a human retrovirus, HTL V. Naturc302:626-628
54 Yoshida M, Miyoshi I, Hinuma Y (1982) Isolation and characterization
of retrovirus from cell lines of human adult T -cell Ieukcmia and
its implication in the disease. Proc Natl Acad Sci USA 79.2031-2034
55. Yoshida M, Seiki M, Yamaguchi K, Takatsuki K (1984) Monoclonal
integration ofhuman T-cell leukemia provirus in all primary tumors
of adult T-cell leukemia suggests causative role of human T-cell
Ieukemia virus in thc disease. Proc Natl Acad Sci USA 81.2534-2537
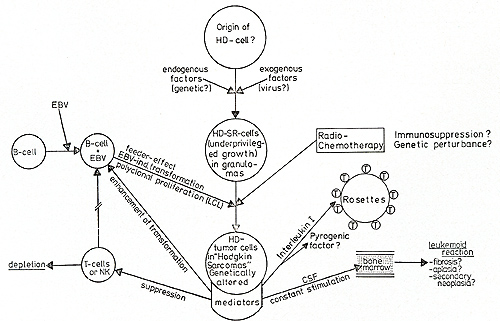
Fig.2. Hypothetic pathogenesis of Hodgkin's disease
undefined cell in normal tissue [ 13, 15], which could be the normal
counterpart of the "malignant" H- and SR-cells (see Stein, this
volume). The pathogenetic mechanisms involved in the transformation
of a normal cell, possibly playing some role in immune and hematopoietic
regulation, is unknown. Endogenous (genetic?) and exogenous (viruses,
chemical agents, both?) might induce a gradual "evoIution" from
a primarily nonproliferating, biologically active cell, which by
its products (CSF, II 1) might create the clinically not very aggressive
"Hodgkin's lymphoma ", to a genetically altered (Fonatsch et al.
unpublished results) more malignant cell, embedded in the histological
entity of a "Hodgkin sarcoma." Radiochemotherapy might act as a
cofactor in this process of gradual malignization. Of the HD patients,
however, 60%-90% are cured by radio- and/ or chemotherapy in the
early stages of this process before genetically altered cells have
chance to commence rapid proliferation and possibly exert resistance
to cytoreductive therapy. The variance in the histological presentation
of Hodgkin's disease could reflect this gradual malignization process:
Paragranuloma and/ or lymphocytic predominance and lymphocyte-enriched
nodular sclerosis would identify a stage of "low risk", with a high
functional activity of the H- and SR-cells, producing mediators
like CSF, Interleukin 1, but still restricted in cellular proliferation.
If cytoreductive therapy is carried out at this stage, cure is possible
in up to 90% of cases ([ 10], Schellong, personal communication).
If the HD cells withstand therapy by either genetically inherent
or resistance mechanisms acquired during treatment, the patient
will present a picture of a more malignant Hodgkin's sarcoma with
a higher number of rapidly proliferating H- and SR-cells. These
cells could still have retained their biological mediator production,
but the balance might be toward more production of immune suppressive
and EBV transformation enhancing factor. The fact that many Hodgkin's
disease patients develop high antibody serum titers against EBV
antigens and give rise to EBVinduced lymphoblastoid cell cultures
significantly more than normal individuals [4] could be explained
not only by T -cell immunosuppression but also by a direct influence
of an EBV transformation enhancing factor. The resulting polyclonal
lymphoblastoid transformation could "feed" or protect the tumor
cell, possibly under a concomitant protection of the rosetting OKT-4-positive
T -helper cells, attaching to the H- and SR-cells. These protection
mechanisms might enable an a priori "lowgrade malignant" HD cell
to "sneak through" to a higher malignant proliferating tumor cell,
which in 15%-20% of the clinical outcome could eventually kill the
patient. Most Hodkgin's disease patients, however, do not die of
tumor cell proliferation, but of biological side effects of immune
deficiency and hematological complications, possibly due to some
of the descri bed factors.
References
1. Borum K (1980) Increasing frequency of acute myeloid leukemia
complicating Hodgkin's disease: A review. Cancer 46: 1247-1252
2. Coltman CA, Dixon DO (1982) Second malignancies complicating
Hodgkin's disease: A southwest oncology group 10-year follow up.
Cancer Treat Rep 66: 1023-1034
3. De Vita VT, Lewis BJ, Rosenzweig Met al. (1978) The chemotherapy
of Hodgkin's disease. Cancer 42: 979-990
4. Diehl V, Johannson B (1977) Establishment of peripherallymphoid
cell cultures from patients with Hodgkin's disease (HD) depending
on Epstein-Barr virus (EBV) reactivity and cellular immunity. Blut
34: 227 -236
5. Diehl V, Kirchner HH, Schaadt Met al. (1981) Hodgkin's disease:
Establishment and characterization of four in vitro cell lines.
J Cancer Res Clin Oncoll01 : 111-124
6. Diehl V, Kirchner HH, Burrichter H, Stein H, Fonatsch C, Gerdes
J, Schaadt M, Heit W, Uchanska-Ziegler B, Ziegler A, Heinz F, Sueno
K (1982) Characteristics of Hodgkin's disease-derived cell lines.
Cancer Treat Rep 66:615-632
7. Fisher RI, Bostick-Bruton F, Diehl V (to be published) Neoplastic
cells obtained from Hodgkin's disease function as accessory cells
for mitogen-induced, human T -cell proliferative responses
8. Fisher RI, Bostick-Bruton F, Sander DN , Diehl V (to be published)
Neoplastic cells obtained from Hodgkin's disease are potent stimulators
of human primary mixed lymphocytic cultures
9. Glicksman HS, Pajak TF, Gottlieb A et al. (1982) Second malignant
neoplasms in patients successfully treated for Hodgin's disease:
A cancer and leukemia group B study. Cancer Treat Rep 66: 1035-1044
10. Kaplan HS (1981) Hodgkin's disease: Biol ogy, treatment, prognosis.
Blood 57:813 11. Longo Dl, Young RC, De Vita VT (1982) The chemotherapy
for Hodgkin's disease: The remaining challenges. Cancer Treat Rep
66:925-936
12. Schaadt M, Diehl V, Stein H et al. (1980) Two neoplastic cell-1ines
with unique features derived from Hodgkin's disease. Int J Cancer
26: 723- 731
13. Schwab U, Stein H, Gerdes J, lemke H, Kirchner HH, Schaadt
M, Diehl V (1982) Production of a monoclonal antibody specific for
Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset
of normal lymphoid cells. Nature 299:65
14. Stein H, Gerdes J, Kirchner HH et al. (1981) Hodgkin's disease.
Imm unohistological analysis of Hodgkin- and Sternberg-Reed cells.
J Cancer Res C1in Onco1101: 125-134
15. Stein H, Gerdes J, Schwab U, lemke H, Mason DY, Ziegler A,
Schienle W, Diehl V ( 1982) Identification of Hodgkin- and Sternberg-Reed
cells as a unique cell type derived from a newly detected cell population.
Int J Cancer 30: 445-459
16. Valagussa P, Santaro A, Kenda R et al. (1980) Second malignancies
in Hodgkin's disease: A complication of certain forms of treatment.
Br Med J 280:216-219
|




