|
A. Introduction Clonal culture systems using semisolid media for the detection of granulocyte-macrophage (Bradley and Metcalf 1966; Pike and Robinson 1970; Pluznik and Sachs 1966), erythroid (Stephenson et al. 1971 ), lymphoid (Fibach et al. 1976; Metcalf et al. 1975a; Sredni et al. 1976) and megakaryocytic (Metcalf et al. 1975b) progenitors have contributed greatly to investigations of hematopoiesis. Unfortunately, such systems possess two major limitations: ( 1) observations are restricted to relati vely short time intervals and (2) the interaction between different kinds of cells cannot easily be studied. More recently, liquid culture systems have also been investigated. Golde and Cline (1973) described the short-term liquid culture of human marrow using an in vitro diffusion chamber in which cells grew both in suspension and on a dialysis membrane. Proliferation and maturation of granulocytes and macrophages in these chambers persisted for 4 weeks. Dexter et al. ( 1973) reported the development of a liquid system for the cocultivation of mouse thymus and bone marrow in which CFUcs (granulocyte-macrophage progenitor cells) were generated for at least 10 weeks and CFUss (pluripotent stem cells) were present for 14 days. The same group (Dexter and Lajtha 1974; Dexter and Testa 1976; Dexter et al. 1977) later developed a method for the long-term culture of mouse bone marrow cells alone in liquid medium. Such cultures produce both CFUcs and CFUss for several months. Hematopoiesis in this system is dependent upon the presence of a marrow-derived adherent population consisting of three cell types : phagocytic mononuclear cells, endothelial cells, and giant lipid-laden adipocytes. Initially, only certain lots of horse serum had the ability to stimulate the growth of these essential adipocytes. Greenberger ( 1978) reported that "deficient" lots of horse serum could be reconstituted with corticosteroids. Long-term liquid culture systems for the cultivation of human bone marrow cells are urgently needed. Moore and Sheridan (1979) reported the establishment of human marrow cultures using conditions similar to those described by Dexter et al., but CFUc production was limited to 6-8 weeks. More recently, Moore et al. ( 1979) reported sustained longterm hematopoiesis in liquid cultures of marrow from a subhuman primate, the tree shrew (Tupaia glis). We now present additional information concerning a recently described method ( Gartner and Kaplan 1980 ) for the long-term culture of human marrow cell populations based on modifications of the murine system.
I. Media Fischer's complete growth medium consisted of Fischer's medium (Gibco #320-1735) supplemented with 10high - 7 M hydrocortisone sodium succinate (Upjohn) and either 25 % horse serum (HoS; Flow Labs), 25% fetal bovine serum (FBS ; Microbiological Associates), or 12.5% each of HoS and FBS. McCoy's complete growth medium consisted of the following: McCoy's 5a medium, modified, (Gibco #430-1500), 10high - 7 M hydrocortisone, 1 % sodium bicarbonate solution (Gibco #670-5080), 1% MEM sodium pyruvate solution (Gibco 3201360), 1% MEM vitamin solution (Gibco #3201120), 0.8%c MEM amino acids solution (Gibco 0#0320-1135), 0.4% MEM nonessential amino acids solution (Gibco 0#0320-1140) 1% L-glutamine, 200mM solution (Gibco 320-5030), 1% penicillinstreptomycin solution, 10,000 u pen/mI, 10,000 mcg strep/ml (Gibco 0#0600-5140), and either 25% HoS, 25% FBS, or 12.5% of each. Only freshly prepared medium was used for the initiation and maintenance of cultures. All serum was heat inactivated at 56°C for 1 h and stored frozen prior to use. In the presence of 10high - 7 M hydrocortisone, all lots of HoS tested were able to support the development of a good stromal layer. Only those lots of FBS which sustained the growth of our fastidious human lymphoma cells (Epstein and Kaplan 1974; Kaplan et al. 1979) were used for these cultures.
Normal marrow specimens were obtained from resected ribs of patients undergoing thoracotomy. Immediately after removal from the patient, the rib was cut into segments 2-3 cm in length and immersed in cold, Ca + + -free Dulbecco's phosphate-buffered saline (PBS; Gibco) supplemented with 1% pen-strep solution and beef lung sodium heparin (Upjohn), 10 µ/ml final concentration. All extraneous connective tissue was cut away from the segments and they were gently rinsed with fresh Ca + + -free Dulbecco's PBS. Care was taken not to flush the marrow from the segments at this step. Using two 6" blunt-ended forceps, the rib segments were pried apart longitudinally. With the tip of a forcep, the marrow was scraped into a lOO mm glass or plastic petri dish containing 20 ml cold complete growth medium (two dishes were used for the marrow from each resected rib.) The medium containing the marrow clumps was then transferred to a 50 ml centrifuge tube (Corning) and pipet ted moderately vigorously to break apart the cell clumps and create a single cell suspension. (In our hands, cultures initiated from single cell suspensions have consistently proven superior to those in which cell clumps were seeded). Viable cell counts were performed using 0.04 %c trypan blue; viability was consistently 98%-100%. Wright-Giemsa-stained slides of the fresh specimens were also prepared to insure that the marrow was normal. Between 1-2 X 109 nucleated cells were usually recovered from a rib. Two X 107 viable nucleated cells in 10 ml of complete growth medium were seeded into plastic T -25 flasks (Corning) or 1 X 107 nucleated cells were seeded into Cluster6 35 mm wells (Costar) containing glass coverslips (Corning.) Cultures were incubated at 33°C in 5%c CO2 in air. (Our early experiments demonstrated the superiority of 33°C over 37°C in terms of supporting long-term hematopoiesis, although 37°C accelerated the development of the stromal layer. In contrast, Moore and Sheridan ( 1979) reported that 37°C was superior for their human marrow cultures.) Weekly feedings consisted of removal of 5 ml spent medium containing non adherent cells and addition of 5 ml fresh medium. The nonadherent cells were counted, used for morphologic and cytochemical studies, and assayed for CFU c. Aspirates of normal sternal marrow were obtained from patients undergoing cardiovascular surgery. Aspirates were also obtained from the iliac crests of patients with acute myeloid leukemia (AML) before treatment, while in remission, and during relapse. These specimens were drawn into heparinized syringes and immediately transferred to tubes containing 2 ml of the previously described complete McCoy's growth medium. Wright's Giemsa-stained preparations of the leukemic aspirates were examined to estimate the seeding density required for the development of the stromal microenvironment in culture. Specimens comprised almost exclusively of myeloid blasts required greater seeding densities for development of the stromal microenvironment. Cell counts were also performed. With the leukemic specimens, between 5 X 106 and 2 X 107 nucleated cells were seeded per T -25 flask, depending on the total number of nucleated cells available. From the normal marrow aspirates, 1-2 X 107 nucleated cells were seeded per T -25 flask. Two to five days after the initial seeding of the aspirate suspensions, the nonadherent cells were removed from each flask independently and separated on individual "mini" Ficoll-Hypaque gradients (Boyum 1968) using 8 ml cell suspension in PBS plus 3 ml Ficoll-Hypaque solution. The cells at the gradient interface were washed three times in Dulbecco's PBS and returned to the original flasks which contained some adherent cells. Aspirate cultures were fed weekly as described for rib-derived cultures.
One million human peripheral blood mononuclear cells were suspended in 1 ml volumes of 0.5% Noble agar (Difco) in McCoy's 5a medium supplemented with 15% FBS as described by Pike and Robinson (1970) and seeded into 35 mm wells of cluster6 plates (Costar). 1 X 105 viable bone marrow cells in 1 ml volumes of 0.3% Noble agar in McCoy's 15 %c FBS medium were overlayed on the 0.5 %c agar layer. Only 0-1-day-old underlayers were used. Clusters and colonies were scored at day 12-14. Clusters contained 20-50 cells and colonies contained more than 50 cells. CFUc values represent the average of at least three cultures, and three wells per culture were assayed.
For morphologic characterization nonadherent cells and coverslip cultures were stained with Wright'sGiemsa; cytochemical tests were also performed for the presence of nonspecific esterase and myeloperoxidase activity (Yam et al. 1971). Table I. Characteristics of the adherent
population 
Table 1 demonstrates that adipocyte-containing confluent stromal
layers could be initiated and maintained for periods of at least
5 months in either Fischer's or McCoy's medium when supplemented
with both horse serum and hydrocortisone. The stromal layers of
very old cultures (greater than 6 months ), especially those grown
in Fischer's medium, were comprised almost exclusively of adipocytes
(Fig. 1 ). Greenberger ( 1979) also reported the induction by corticosteroids
of lipogenesis in adipocytes in human marrow cultures. In the absence
of horse serum, either medium, despite supplementation with hydrocortisone
and FBS, failed to support the development of significant numbers
of lipid-Iaden adipocytes. In such cultures almost no nonadherent
cells were recovered after 6-8 weeks. Cultures grown in McCoy's
medium supplemented with FBS with or without HoS developed confluent

stromal layers earlier than those supplemented with HoS alone or
grown in Fischer's medium. Fischer's growth medium supplemented
with hydrocortisone and 25% HoS or 12.5% HoS and 12.5% FBS was able
to support the generation of CFUc-forming cells for up to 12 weeks
in some cases. However, the number of colony-forming cells recovered
from such cultures was usually less than 50 per 105 cells assayed.
In contrast, McCoy's complete growth medium supplemented with both
FBS and HoS not only provided adequate nutrition for the early development
and maintenance of stromal layers containing abundant adipocytes
but also contributed either directly or indirectly (through the
stromal microenvironment) to longterm hematopoiesis and significantly
greater yields of CFU c. Figure 2 illustrates the appearance of
a typical culture at day 14. A confluent stromal layer can be seen
underneath the nonadherent cells. A most important feature of these
long- term cultures is the presence of "cobblestone"-like areas,
presumably regions of hematopoiesis from which the nonadherent cells
arise. Figures 3 and 4 illustrate such cobblestone-like areas in
cultures at 6 weeks and 16 weeks, respectively. At higher magnification
(Fig. 5), the flattened polygonal cells in such areas are seen to
be so tightly packed together that their boundaries may be difficult
to discern. With time some of these cells become more rounded granules
can be seen to appear within their cytoplasms and ultimately they
become nonadherent. When coverslip cultures were gently washed extensively
to remove non adherent cells and stained for myeloperoxidase activity,
it was observed that cobblestone areas did indeed contain both myeloid
and monocytoid cells. Coverslip cultures stained for nonspecific
esterase activity revealed monocytoid cells scattered throughout
the stromal layer , in islands of hematopoiesis, and sometimes in
large, tight clusters within the stromal layer. In general, nonspecific
esterase-positive cells were much more intensively stained outside
of the cobblestone areas. The kinetics of the generation of cells
with colony-forming ability in these long-term cultures appeared
to follow two different patterns, designated the "hyperproliferative"
and "homeostatic" patterns. The hyperproliferative pattern describes
cultures in which the  Fig. 2. A human marrow culture at 14 days. X 81  Fig. 3. A human marrow culture at 6 weeks illustrating adipocytes and cobblestone areas of active hematopoiesis. X 50  Fig. 4. A human marrow culture at 16 weeks illustrating a cobblestone area. X 77 

were mature macrophages. This finding is in agreement with that of Moore and Sheridan (Moore and Sheridan 1979; Moore et al. 1979) in long-term human marrow cultures grown in Fischer's medium with HoS. With Fischer's medium plus 25% HoS or 12.5 % HoS and 12.5% FBS, some immature monocytoid and both immature and mature myeloid cells were also present but in small numbers. In contrast, cultures grown in McCoy's FBS plus HoS growth medium contained many more immature monocytoid and myeloid cells as well as mature myeloid cells. Spontaneous mitotic figures were also seen in stained preparations of these non adherent cells. The numbers of myeloid cells often exceeded the numbers of mature macrophages in the McCoy's FBS plus HoS cultures, unlike the situation in Fischer's medium. In younger cultures (less than 6-8 weeks) monocytoid and myeloid cells at various stages of differentiati on were generally observed, and fewer differences were detected between the different growth media. Aspirates of normal sternal marrow taken from patients during cardiovascular surgery and of leukemic marrow from the iliac crests of AML patients were also cultured. The probability of successful establishment of cultures from such specimens appeared dependent upon the quality of the aspirate and, in the case of the leukemic specimens, the previous medi Table 2. Characteristics of the non adherent population
  Fig.7. Agar culture colony arising from a CFUc harvestet from a 14-week-old-human marrow culture in liquid medium. X 19  Fig. 8. Nonadherent population from a 9-week-old human marrow culture. X 310 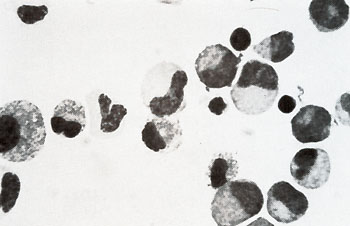 Fig.9.Nonadherent population from a 12-week-old human marrow culture. X 324  Fig. 10. A I-month-old human marrow culture from an aspirate from AML patient T. H. X 50 
It has been demonstrated that hematopoiesis in long-term liquid cultures of murine (Allen and Dexter 1976) and Tupaia glis (Moore et al. 1979) marrow is dependent upon the presence of lipid-laden adipocytes. Our results suggest that this is also the case for human marrow. Horse serum in conjunction with hydrocortiso ne appears to be essential for the initial growth and/ or differentiation of the adipocytes. In limited studies cultures grown in the presence of freshly collected pooled human serum and 10high - 7 M hydrocortisone, with or without the addition of FBS, failed to develop mature adipocytes. The numbers of lipid-containing adipocytes in cultures from different specimens varied tremendously, especially in younger cultures (less than 8 weeks). Interestingly, some cultures established from AML marrow aspirates often possessed extremely large numbers of adipocytes within 2 weeks after initiation of the cultures. In contrast, occasionally other AML marrow cultures completely failed to develop any adipocytes. In those cases where more than 1 aspirate was obtained at different time intervals from the same AML patient considerable variation was also seen between specimens with regard to the numbers of adipocytes observed in the cultures, without any evident correlation with the patient's clinical condition or the status of the disease. The culture of additional leukemic specimens may elucidate this problem. The cobblestone areas were often not seen immediately adjacent to the adipocytes, especially in younger cultures. This suggests that direct cell-to-cell contact between granulocytemacrophage committed progenitors and well-differentiated adipocytes may not be required for the maturation of the progenitors. It seems clear that the successful maintenance of long-term hematopoiesis in these cultures is crucially dependent upon the development and preservation of an intact, competent stromal layer. Factors which affect the integrity of the adherent layer, such as increased acidity of the culture medium or sometimes the addition of an extraneous cell population to the culture, resulted in a decrease in hematopoiesis as measured by CFUc production. The regeneration of the stromal population in the original culture vessel has not occurred to any significant degree in our cultures. (This is unlike the situation reported for cultures of tree shrew marrow where hematopoiesis was restored along with the regeneration of the stromal layer [Moore et al. 1979]). Thus, great care must be taken to preserve the integrity of the stromal micro-environment. Our recent efforts to subculture stromal populations have met with limited success. When adherent cells trypsinized from old cultures of normal marrow were reseeded into new culture vessels, we have again observed the growth of an adherent population to confluence. Lipid-Iaden adipocytes are lost during trypsinization, so that subcultured layers are initially comprised only of large, polygonal or fibroblastoid cells and macrophages. In some cases, lipid-containing cells have appeared some time later in these cultures, suggesting that adipocyte precursors are trypsinizable or that the lipid droplets are lost during trypsinization and require considerable time to be regenerated. The restoration of long-sustained hematopoiesis in such trypsinized cultures has been generally unsuccessful, though cobblestone areas have occasionally been observed. Trypsinization has proven valuable in generating confluent stromal layers in cultures from AML patients. With some such specimens, very few adherent cells ( other than macrophages) were present in the cultures and confluence of an adherent population was never reached. Such cultures were later trypsinized and the recovered cells seeded at higher cell densities. Confluence of an adherent layer was thus ultimately obtained in many cases. An additional problem in the culture of leukemic specimens was the lack of an appropriate assay system to monitor granulopoiesis in these cultures. In the case of some of the specimens from patients in remission, the non adherent cells recovered from the cultures did not form colonies in soft agar, although such nonadherent cell populations morphologically resembled those recovered from cultures of normal marrow. We believe that both the hyperproliferative and homeostatic patterns described for CFU c production reflect the actual de novo generation of granulocyte-macrophage progenitor cells in the cultures. It appears that the numbers of CFUc detected over time in such cultures exceed the numbers of granulocytemacrophage committed progenitor cells initially seeded. Moreover, serial CFUc assays would have been expected to reveal a rapid depletion of CFUc as weekly samples were withdrawn, if CFUc were merely persisting rather than proliferating in the cultures. This interpretation is further supported by the observation of mitotic figures and by the fact that production of CFU c did not begin to increase in the hyperproliferative pattern until about 4 weeks. Myeloperoxidase-stained preparations have demonstrated that cobblestone areas do contain myeloid cells. The alternative possibility that granulocyte-macrophage committed progenitors remain dormant and undetectable within the stromal matrix for long periods of time until they receive appropriate signals for proliferation and maturation or until they are sloughed off as the culture ages seems less likely in the face of these observations. The differences between the two growth patterns may be attributable to age and other constitutional factors affecting individual hematopoietic activity; they may have been more readily apparent because most of our rib specimens came from older patients. Greenberger et al. ( to be published) have demonstrated that the capacity for long-term hematopoiesis in murine marrow cultures differs considerably from strain to strain. Studies of the stromal microenvironment of these cultures are currently underway which may provide abetter understanding of the source of CFUcs. Sampling problems have been encountered during attempted recovery of nonadherent cells from these cultures. The nonadherent cells tend to .'hover" closely over the stromal layers. Phase contrast observations immediately following feeding of the cultures reveal that even with gentle agitation of the culture vessel, large numbers of non adherent cells remain close to the stromal layer .Possibly the viscosity of the growth medium enhances this effect. Therefore, we believe that the numbers of nonadherent cells harvested in the aliquots of spent medium may not accurately reflect the numbers of non adherent cells present in the cultures. More vigorous agitation of the culture vessel results in some destruction of the delicate stromal microenvironment and cessation of hematopoiesis. We are presently attempting to improve our methods of quantitation. Erythroid (BFU-E) (Testa and Dexter 1977) and megakaryocytic (CFU-M) (williams et al. 1978) progenitors are also produced in long-term murine cultures. The persistence for at least 16 weeks of pre- T cells in murine cultures has also been recently reported (Jones- Villeneuve et al. 1980). To date we have not attempted to assay for such populations in our cultures of human marrow. However, we have established three permanent, polyclonal cell lines of Epstein-Barr virus transformed B-lymphoblastoid cells from marrow cultures of different nonleukemic specimens. All three arose from what appeared to be hematopoietically exhausted stromal layers, one developing at 3 months, one at 4 months, and one at 6 months after initiation of the cultures. All were derived from cultures in Fischer's medium with 25% HoS and hydrocortisone. In the case of the 6-month-old stromal layer, no cobblestone areas remained, and no nonadherent cells had been recovered for 2 months prior to the appearance of the B cells. Moore and Sheridan (1979) reported the conversion of 10% of their human cultures to a lymphoblastoid morphology by the 6th week of culture. We are at present uncertain as to whether our observations represent the generation of B cells from immature precursors in these old cultures or the delayed outgrowth of long-lived EBV-transformed mature B cells. Given the complexity of the stromal microenvironment, it is quite possible that small numbers of mature B cells could have survived undetected. Methods for the culture of human bone marrow are essential for furthering our understanding of normal hematopoiesis and of hematopoietic disease states as well as the effects of therapeutic regimens on marrow subpopulations. Although there are still many improvements to be made in the application of the liquid phase murine culture system to human marrow, we believe the modifications we have described allow for the routine establishment of such cultures in a reproducibly successful way.
This work was supported by research contract N01-CP-91044 from the National Cancer Institute, National Institute of Health, U.S. Dept. of Health, Education, and Welfare (DHEW). Suzanne Gartner is the holder of a predoctoral traineeship in a Cancer Biology Training Program supported by training grant CA-09302 awarded by the National Cancer Institute, DHEW. WethankDr.JamesB.D. Mark for providing the rib specimens, Dr. Stuart Jamieson for providing the normal sternal marrow aspirates, and Drs. Peter Greenberg, Richard Hornes, Robert Feiner, and Ms. Betty Reitsma for providing the leukemic marrow specimens. We are also grateful to Ms. Glenda Garrelts for the nonspecific esterase tests, Dr. Werner Henle for the EBNA determinations, and Dr. Joel Greenberger for helpful discussions.
- Allen TD, Dexter TM (1976) Cellular Interrelationships during in vitro Granulopoiesis. Differentiation 6: 191-194 - Bradley TR, Metcalf D (1966) The growth of mouse bone marrow cells in vitro. Aust J Exp BioI Med Sci 44:2H7-300 - Böyum A ( 196H) I solation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest [Suppl 21] 77: -Dexter TM, Lajtha LG (1974) Proliferation of hematopoietic stem cells in vitro. Br J Hematol 28: 525-530 - Dexter TM, Testa NG (1976) Differentiation and proliferation of hematopoietic cells in culture. In: Methods in cell biology, vol 14. Academic Press, New York, pp 387-405 - Dexter TM, Allen TD, Lajtha LG, Schofield R, Lord BI (1973) Stimulation of differentiation and proliferation of hematopoietic cells in vitro. J Cell Physiol 82 :461-470 - DexterTM, AllenTD, Lajtha LG (1977) Conditions controlling the proliferation of hematopoietic stem cells in vitro. J Cell Physiol 91 :335-344 - Epstein AL, Kaplan HS (1974) Biology of the human malignant lymphomas. I. Estab]ishment in continuous cell culture and heterotransplantation of diffuse histiocytic lymphomas. Cancer 34: 1851-1872 - Fibach E, Gerassi E, Sachs L (1976) Induction of colony formation in vitro by human lymphocytes. Nature 259:127-129 - Gartner S, Kaplan HS (1980) Long-term culture of human bone marrow cells. Proc Natl Acad Sci USA 77:4756-4759 - Greenberger JS (1978) Sensitivity of corticosteroid-dependent insulin-resistant lipogenesis in marrow preadipocytes of obese diabetic (db/db) mice. Nature 275 :752-754 - Greenberger JS ( 1979) Corticosteroid-dependent differentiation of human marrow preadipocytes in vitro. In Vitro 15:823-828- Greenberger JS, Sakakeeny MA, Berndtson K ( to be published) Longevity of hematopoiesis in mouse long-term bone marrow cultures demonstrates significant mouse genotypic variation. J Supramol Struct -Golde D, Cline M (1973) Growth of human bone marrow in liquid culture. Blood 41: 45-57 - J ones- Villeneuve EV, Rusthoven JJ, Miller RG, Phillips RA (1980) Differentiation of Thy 1-bearing cells from progenitors in long-term bone marrow cultures. J Immunol 124:597-601 - Kaplan HS, Goodenow RS, Gartner S, Bieber MM (1979) Biology and virology of the human malignant lymphomas: 1st Milford D. Schultz Lecture. Cancer 43: 1-24 - Metcalf D, Warner NL, Nossal GJV, Miller JFAP, Shortman K, Rabellino F (1975a) Growth of B lymphocyte colonies in vitro from mouse lymphoid organs. Nature 255: 630-632 - Metcalf D, MacDonald HP, Odartchenko N, Sordat LB ( 197 5b ) Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci USA 72: 1744-1748 - Moore MAS, Sheridan A PC (1979) Pluripotent stem cell replication in continuous human, prosimian and murine bone marrow culture. Blood Cells 5:297-311 - Moore MAS, Sheridan A PC, Allen TD, Dexter TM (1979) Prolonged hematopoiesis in a primate bone marrow culture system: characteristics of stem cell production and the hematopoietic microenvironment. Blood 54:775-793 - Pike B, Robinson W (1970) Human bone marrow colony growth in agar-gel. J Cell Physiol 76: 77-84 - Pluznik DH, Sachs L ( 1966) The induction of clones of normal mast cells by a substance from conditioned medium. Exp Cell Res 43:553-563 - Sredni B, Kalechman Y, Michlin H, Rozenszajn LA (1976) Development of colonies in vitro of mitogen-stimulated mouse T lymphocytes. Nature 259: 130-132 - Stephenson JR, Axelrad AA, McLeod DL, Shreeve MM ( 1971 ) Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci USA 68: 1542-1546 - Testa NG, DexterTM (1977) Long-term production of erythroid precursors (BFU) in bone marrow cultures. Differentiation 9: 193-195 - Williams N, Jackson H, Sheridan A PC, Murphy MJ, Elste A, Moore MAS (1978) Regulation of megakaryopoiesis in long-term murine bone marrow cultures. Blood 51:245-255 - Yam LT, Li CY, Crosby WH (1971) Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol 55:283-290 |