|
Medical Department Brookhaven National Laboratory
Upton, New York 11973 The intent of this presentation is to present a notion that the
in vitro bone marrow and blood culture system does not truly measure
the dimensions of the committed stem cell pool. The measure of stem
cell reserve -preferably the pluripotent and the committed stem
cell pools is not a trivial, clinical problem. In the course of
disease, chemotherapy, radiotherapy, one could much better guide
management if there were a reliable reproducible method to assay
for the stem cell reserve. In addition, a stem cell assay for man
is critical to an understanding of normal hematopoiesis and its
full characterization. At first look, the in vitro bone marrow culture
combined with tritiated thymidine suicide appeared to be an answer
to the problem. This can now be questioned. The diffusion chamber
culture technique measures both pluripotent and committed stem cells
but it also is capricious and poorly reproducible although some
technical progress is being made (1,2). The concept of stem cells
in hemopoiesis is several decades old. Maximow and Bloom (3) have
expressed their thoughts clearly. They divided hemopoiesis into
homoplastic and heteroplastic hemopoiesis. Homoplastic hemopoiesis
is defined as the production of mature cells by young elements of
the same type. They believe that under physiological conditions
the needs of the adult organism are supplied generally by homoplastic
hemopoiesis. When requirements for blood cells were increased, as
after hemorrhage, during infection, during regeneration from injury,
homoplastic hemopoiesis was insufficient and new erythroblasts and
myelocytes then developed from a pluripotent stem cell and this
they called heteroplastic hemopoiesis. Modern parlance divides the
stem cells into the pluripotent and the committed stem cells (4,
5). Pluripotent hemopoietic stem cell can only be studied directly
in the mouse. It is measured by the capability of hemopoietic cell
suspensions to produce splenic colonies. The cell that produces
the colony is called the CFU-S. The CFU-S derived from the bone
marrow and spleen of the mouse has a capabilityof producing erythrocytic,
granulocytic, and megakaryocytic lines, respectively (6). Becker
et al. (7) have clearly shown that colonies are formed from a single
CFU-S, demonstrating the clonal nature of the spleen colonies. Till
et al. (8) in studying the growth rates of splenic colonies developed
a stochastic model for stem cell proliferation, in which they feel
that the birth process or replication of the stem cell and the death
process ( differentiation into a cell no longer able to replicate
the CFU-S) appear at random in the population of the colony forming
cells proliferating in the splenic colony. Becker et al. (9) have
determined the fraction of the CFU-S that are in DNA synthesis by
treating suspensions of stem cells with high concentrations of ³HTdR
to kill the cells in DNA synthesis. When the hemopoietic system
is expanding a large fraction of the CFU-S (as much as 60-700/0)
may be in DNA synthesis. In the steady state proliferation of the
adult marrow and spleen the fraction of CFU-S in DNA synthesis is
barely perceptible. Vassort et al. (10) have shown that the CFU-S
has a ³HTdR suicide varying from 9-200/0 depending upon the strain
of the adult mouse. Onlya fraction of the CFU-S produce splenic
colonies upon transplantation. The fraction of these that produce
splenic colonies is called the f-factor (11) ; this is roughly 0.17.
There is considerable variation in the f-factor depending upon the
period of time that the cell will circulate in the blood and many
other factors of biological and statistical nature that may operate
at any given time. As of the moment there is no way of detecting
the pluripotent stem cell in man or mammals other than the mouse,
and to a lesser extent, the rat. Pluripotent stem cells migrate
through the blood. Two clear-cut experiments showed this years ago.
Brecher and Cronkite (12) showed that when one member of a parabiotic
pair is shielded while the other is receiving fatal irradiation,
the irradiated one is protected from radiation lethality, thus showing
the migration of the pluripotent hemopoietic stem cell from the
nonirradiated into the irradiated twin. Swifl: et al. (13) showed
the protection against radiation is conferred if one half the body
only is exposed, followed in a matter of minutes by exposure of
the remaining half and shielding of the previously exposed portion,
thus showing migration of the pluripotent hemopoietic stem cell
during this interval. Goodman and Hodgson (14) and Trobaugh and
Lewis (15) showed that the PHSC circulate in the peripheral blood
under normal steady state conditions. In mice the concentration
of the CFU-S is 10-30 cells/mI (16). Their half-time in the blood,
however, is reported to be only about 6 minutes (17). Accordingly,
one can estimate in the 30 g mouse that the PHSC daily turnover
rate is equal to: 
STRUCTURE OF HUMAN BONE MARROW HEMOPOIETIC CELL
PROLIFERATION 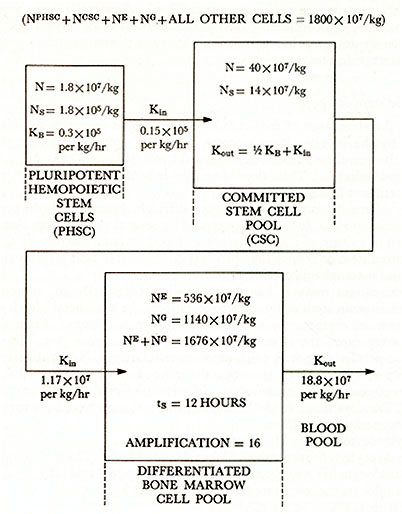
1. Boyum, A., Carsten, A. L., Laerum, 0. D. and Cronkite, E. P. Kinetics of celJ proliferation of murine bone marrow cells cultured in diffusion chambers: effect of hypoxia, bleeding, erythropoietin injections, polycythemia, and irradiation of the host. Blood 40:174, 1972. 2. Cronkite, E. P., Boecker, W., Carsten, A. L., Chikkappa, G., Joel, D., Laissue, J. and Öhl, S. The use of diffusion chamber cultures in the study of normal and leukemic cell proliferation in man. In Robinson, W. A. (ed.) Hemopoiesis in Culture, DHEW Publ. No. (NIH) 74-205, Bethesda, 1973, P. 185. 3. Maximow, A. A. and Bloom, W. (eds) Text Book of Histology, Philadelphia, W. B. Saunders, 1935. 4. Stohlman, F., Jr. The Kinetics of Cellular Proliferation, Grune and Stratton, New York, 1959. 5. Stohlman, F., Jr. (ed) Symposium on Hemopoietic Cellular Proliferation. St Elizabeth's Hospital Centennial 1869-1969, Boston, Massachusetts, Nov. 5-6 1969, Grune and Stratton, New York, 1970. 6. Till, J. E. and McCulloch, E. A. A direct measurement of the radiation sen sitivity of normal mouse bone marrow cells. Radiat. Res. 14:213, 1961. 7. Becker, A. J ., McCulloch, E. A. and Till, J. E. Cystological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197:452, 1963. 8. Till, J. E., McCulloch, E. A. and Siminovitch, L. A. stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc. Nat. Acad. Sci. 51 :29, 1964. 9. Becker, A. J., McCulloch, E. A., Siminovitch, L. and Till, J. E. The effect of differing demands for blood cell production on DNA synthesis by hemopoietic colony-forming cells of mice. Blood 26:296, 1965. 10. Vassort, F., Winterholer, M., Frindel, E. and Tubiana, M. Kinetic parameters of bone marrow stem cells using in viva suicide by tritiated thymidine or by hydroxyurea. Blood 41 :789, 1973. 11. Till, J. E. and McCulloch, E. A. The "f-factor" of the spleen colony assay for hemopoietic stem cells. Series Hematology 5:15-21,1972. 12. Brecher, G. and Cronkite, E. P. Postradiation parabiosis in survival in rats. Proc. Soc. Exper. BioI. Med. 77:292, 1951. 13. Swift, M. N., Taketa, F. T. and Bond, V. P. Regionally fractionated x-irradia tion equivalent in dose to total body exposure. Rad. Res. 1 :241, 1954. 14. Goodman, J. E., Hodgson, G. S. Evidence for stem cells in the peripheral blood of mice. Blood 19:702, 1962. 15. Trobaugh, F. E., Lewis, J. P., Jr. Repopulating potential of blood and marrow. J. Clin. Invest. 43:1306,1964. 16. Hellman, S. and Grate, H. E. Kinetics of circulating haemopoietic colonyforming units in the mouse. In Doyle, E. (ed.) Effects of Radiation on Cellular Proliferation and Differentiation. Vienna, International Atomic Energy Agency, 1968(a), p. 187. 17. Hodgson, G., Guzman, E. and Herrera, C. Characterization of the stem cell population of phenylhydrazine-treated rodents In: Doyle, E. (ed.) Effects of Radiation on Cellular Proliferation and Differentiation. Vienna, International Atomic Energy Agency, 1968, p. 163. 18. Senn, J. S. and McCulloch, E. A. Radiation sensitivity of human bone marrow cells measured by a cell culture method. Blood 35 :56, 1970. 19. Moore, M. A. S. and Williams, N. Functional, morphologic, and kinetic analysis of the granulocyte-macrophage progenitor cell. In Robinson, W. A. (ed) Hemopoiesis in Culture, DHEW Publication No. (NIH) 740205, Bethesda, 1973, p 17. 20. Cronkite, E. P. and Vincent, P. C. Granulocytopoiesis. Ser. Haemat. VoI. II, 4, 1969, 3-43. 21. Cartwright, G. E., Athens, J. W. and Wintrobe, M. M. The kinetics of granu lopoiesis in normal man. Blood 24 :780, 1964. 22. Donohue, D. M., Reiff, R. H., Hanson, M. L., Betson, Y and Finch, C. A. Quantitative measurements of the erythrocytic and granulocytic cells of the marrow and blood. J. Clin. Invest. 37:1571,1958. 23. Donuhue, D. M., Gabrio, B. W. and Finch, C. A. Quantitative measurement of hematopoietic cells of the marrow. J. Clin. Invest. 37:1564,1958. 24. Stryckmans, P., Cronkite, E. P., Fliedner, T. M. and Tamos, J. DNA synthesis time of erythropoietic and granulopoietic cells in human beings. Nature 211:717, 1966. 25. Odartchenko, N., Cottier, H., Feinendegen, L. E. and Bond, V. P. Evaluation of mitotic time in viva using tritiated thymidine as a cell marker: Successive labeling with time of separate mitotic phases. Exp. Cell. Res. 35 :402, 1964. 26. Killmann, S. A., Cronkite, E P., Fliedner, T. M. and Bond, V. P. Mitotic index of human bone marrow cells I. Number and cytologic distribution of mitoses. Blood 19:743,1962. |