|
|
|
Max-Planck-lnstitut für experimentelle Medizin,
Abteilung Molekulare Biologie Recent findings of viral specific RNA in human tumors such as human breast cancer (1), leukemias (2), sarcomas (3) and lymphomas (4) which are related to RNA of RNA tumor viruses of animals support the idea of a virus aetiology in human cancer. The establishing of cell cultures of human origin transformed in vitro should provide a powerful aid in the search for human cancer. Diseases which are related to leukemia and which can be considered as being preleukemic stages seem to be ideal systems to study the malignant transformation in man. In addition the disease to be studied should be reasonably frequent and the disease entity well characterized. Furthermore, during the course of the disease some similarities should occur between the disease and experimental RNA tumor virus induced diseases in animals. This seems to be true for Polycythaemia vera (PV). PV is a proliferative disease of the hematopoietic system, mainly of the erythropoietic compartment. Often PV changes, expecially after irradiation and chemotherapeutical treatment, to acute leukemia, chronic myelogenic leukemia or myeloid metaplasia (5 ). In the development of the Friend virus induced leukemia in mice a similar polycythemic stage is observed ( 6) .In the Friend polycythemia as well as in PV, an unregulated multiplication, but normal differentiation of the cells of the red compartment is observed. In both diseases the abnormal erythropoietic precursor cells do not respond to the proteohormone erythropoietin. We have shown that Friend virus transformed erythropoietic tissue culture cells also do not respond to erythropoietin (7). For these reasons bone marrow cells of patients with Polycythaemia vera seem to be an appropriate material for establishing cultures to study processes leading to leukemic transformation in man.
Bone marrow of a 69-year old male patient with PV has been cultivated. After a proliferative phase of two weeks the cells successively became fibroblastoid and formed monolayers. After 8 weeks the contact inhibited monolayer cultures did not contain cells in suspension. During the ninth week a "piling up" has been observed on different loci of the monolayers of two parallel cultures. The spontaneously transformed cells grow in suspension as single cells or in clusters. They have a generation time of about 24 hours. Figure 1 shows the possible correlation between the time course of the cell culture and the clinical course of the disease. Cytologically there was no difference between the cell types of the culture and normal bone marrow during the first 3 weeks. After the transformation, however , 90 % of the cells are blasts, 8 % orthochromatic erythroblasts and a small portion lymphoid cells (see Table 1).
The cytological observations of orthochromatic erythroblasts led
to biochemical investigations for globin synthesis, although the
cells gave a benzidine negative reaction. Cells were labelled with
3 H- and 14 C-Ieucine and globin chains were isolated (8,9). Radioactively
labelled tissue culture protein eluted as defined peaks together
with the a and ß carrier globin. Tryptic fingerprint analysis of
alfa - and ß-chains were carried out as described (10). Preliminary
results show that alfa - and ß-type peptides are present. Stimulation
with 1 % DM50 for 5 days had no effect on globin synthesis contrary
to the Friend virus induced erythroleukemic mouse cells in culture
( 10) . 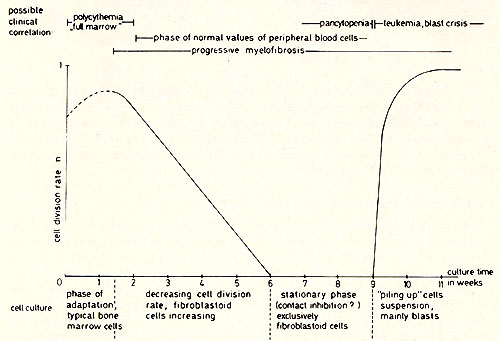 Fig. 1: Schema tic representation of changes leading to PV tissue culture and possible clinical correlation.
Table 1: Cytological and cytochemical
classification of the cells during the process of establishing the
cell culture 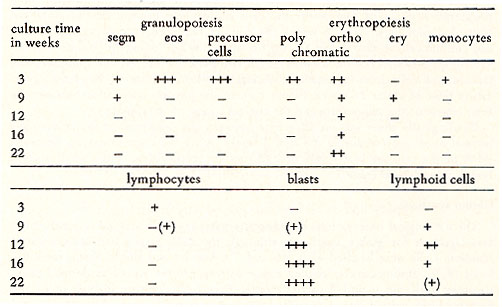 Karotype The karyotype is hyperdiploid. All cells ( n 20) contained 48 chromosomes. The 2 additional chromosomes appear as one large metacentric and one large submetacentric chromosome (see Figures 2 and 3 ). Furthermore, a change in one chromosome of the F-group ( deletion or inversion) can be observed. This anomaly is considered to be a specific aberration for some cells in several patients with PV (11 ). High molecular weight RNA and reverse transcriptase The possible role of the RNA dependent DNA polymerase as a key enzyme in neoplastic transformation of human cells has guided several investigations since the discovery of the enzyme in RNA tumor viruses (12,13). For the simultaneous detection of high molecular weight RNA and reverse transcriptase in PV cells the method of 5CHLOM and 5PIEGELMAN (14) was applied. It was found earlier that the initial DNA product of the reaction of reverse transcriptase on 705 RNA is complexed via hydrogen bonds to the template. This was demonstrated by the unusual position of DNA product in glycerol velocity and Cs2504 equilibrium gradients. After mild heat denaturation the DNA product was detected in the expected positions in CS2 504 and glycerol gradients (15, 16). 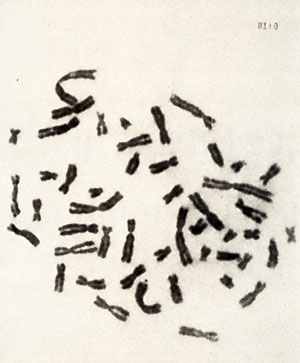 Fig. 2: Metaphase chromosomes of a PV cell 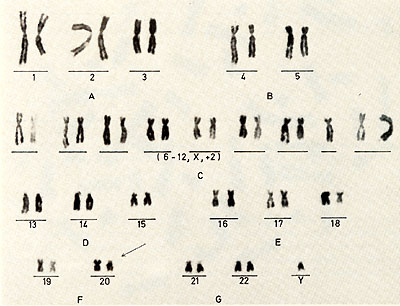 Fig.3: Idiogram of the chromosomes shown in fig.2, Arrow indicates the changed F-group chromosome 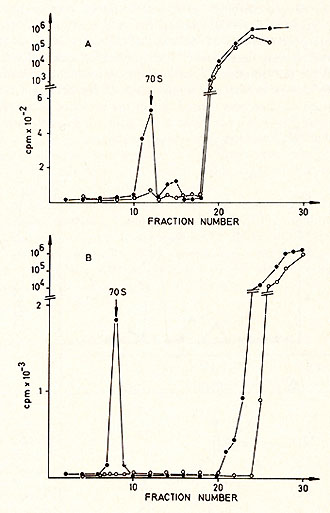
inhibit DNA dependent DNA synthesis. The reaction was stopped
by SDS treatment, deproteinized with phenol-cresol and centrifuged
in a 15-30% glycerol gradient with a size marker (Figure 4 ). The
radioactive moiety of the 70S region was subjected to CS2 SO4 equilibrium
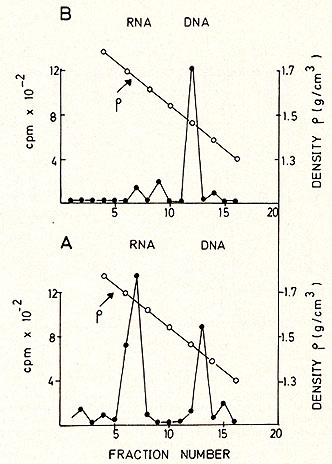
density centrifugation (Fig. 5 ). Most of the radioactivity is found
in the RNA region (1.64-1.68 g/ml), although some material is found
in the DNA region, probably due to a breakdown during the isolation
procedure (Fig. 5a). After alkaline hydrolysis the same material
bands in the DNA region (Fig. 5b ). Table 2. Template response of PV DNA polymerase 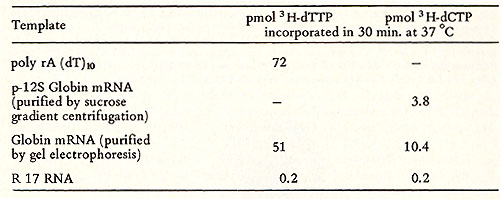 Electronmicroscopy
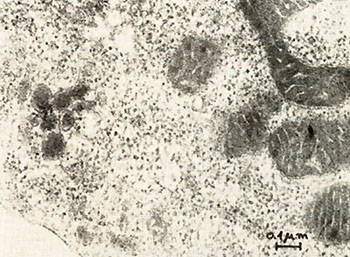
Fig. 6: Ultrathin section of a PV-cell. The cell was fixed with 2.5% glutaraldehyde in 0.1 M potassium-sodium-phosphate-buffer, pH 7.2, followed by postfixation with 1 %I{; osmiumtetroxide in isotonic sodium-veronal-acetate buffer, pH 7.2 and with 0.5 % uranylacetate in 0.1 M acetate buffer, pH 3.9. The specimens were dehydrated with ethanol and embedded in epon. Sections were stained with 3 % aqueous uranyl-acetate and 0.3 % Iead-citrate. Photographs were taken in a JEOL J em lOO B electron-microscope at 80 K V. Mag. : 80,000 x 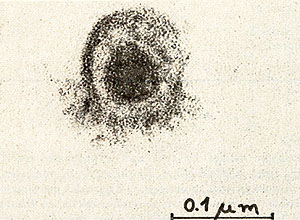
We have established a cell culture line from bone marrow of a patient with a Polycythaemia vera (PV) disease. During the time course of the establishing of the culture we observed a spontaneous transformation in vitro. Cytologically no difference was found between cell types of the culture and normal bone marrow during the first 3 weeks. After the transformation, however, 90% of the cells are blasts, 8 % orthochromatic erythroblasts and a small portion lymphoid cells. The karyotype of the cells in culture is hyperdiploid and contains 48 chromosomes. Furthermore, a change in one chromosome of the F-group (inversion or deletion) is observed. The cells produce adult human globin and contain particles with high molecular weight RNA and reverse transcriptase activity. Investigations by electron microscopy revealed virus like particles.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (G. G., J. K., N. K., W. 0.).
1. Axel, R., Schlom, J., Spiegelman, S., Nature 235,32 (1972) 2. Hehlmann, R., Kufe, D., Spiegelman, S., PNAS (USA) 69,435 (1972) 3. Kufe, D., Hehlmann, R., Spiegelman, S., Science 175,182 (1972) 4. Hehlmann, R., Kufe, D., Spiegelman, S., PNAS (USA) 69,1727 (1972) 5. Modan, B., "The Polycythemic Disorders", C. C. Thomas Publ., Springfield, Illinois, USA (1971) 6. Mirand, E. A., in "Regulation of Hematopoiesis", ( edit. by A. S. Gordon), Vol. 1,635 (1970), (Appleton-Century-Crofts) 7. Ostertag, W ., Kluge, N., Melderis, H., Steinheider, G., Gaedicke, G., Dube, S., Verhandl. der 79. Tagung der Dt. Gesellschaft f. Innere Medizin, 1973, in press 8. Borun, T. W ., Scharff, M. D., and Robbins, E., Biochim. Biophys. Acta 149, 302 (1967) 9. Clegg, J. B., Naughton, M. A., and Weatherall, P. J ., Nature 207, 945 (1965 ) 10. Ostertag, W., Melderis, H., Steinheider, G., Kluge, N., Dube, S., Nature NB 239, 231 (1972) 11. Reeves, B. R., Lobb, D. S., Lawter, S. D., Humangenetik 14,139 (1972) 12. Temin, H. M. and Mizutani, S., Nature 226, 1211 (1970) 13. Baltimore, D., Nature 226, 1209 (1970) 14. Schlom, J. and Spiegelman, S., Science 174,840 (1972) 15. Spiegelman, S., Burny, A., Das, M. R., Keydar, J ., Schlom, S., Travnicek, M. and Watson, K., Nature 227, 563 (1970) 16. Rokutanda, M., Rokutanda, H., Green, M., Fujinaga, K., Ray, R. K. and Gurgo, C., Nature 227, 1026 ( 1970) |