|
The Department of Medicine, St. Elizabeth's Hospital
and Tufts Medical School, Boston, Massachusetts Before considering the problem of leukemia it seems appropriate
to briefly review the normal regulation of hemopoiesis. I shall
confine my remarks to normal myelopoiesis and those leukemias associated
with disorders of myelopoiesis i. e. acute myelocytic, progranulocytic
and undifferentiated stem cellleukemias as well as chronic myelocytic
leukemias. A schematic outline of normal myelopoiesis (1) is shown
in figure 1. It is to be noted that the myeloid system shares a
common precursor cell with the erythroid aand megakaryocytic series.
This common precursor cell is referred to as the pluripotent hemopoietic
stem cell. Intermediate between the pluripotent stem cell and those
recognizable myeloid elements, which may be identified under the
light microscope, is a compartment which is committed to myelopoiesis.
Similar compartments exist for the erythroid and megakaryocytic
series. There is evidence) which has recently been reviewed ( 1),
that suggests that monocytes and myeloid elements derive from a
common committed precursor cell in which case the committed myeloid
stem cell compartment can not be considered truly unipotent. Further
the myeloblast and progranulocyte differentiates along one of three
lines the neutrophil, eosinophil or the basophil. We do not know
at what stage this commitment is made i. e. committed stem cell,
myeloblast or progranulocyte, nor what is the decision making mechnism.
In animals there is a technique to evaluate the pluripotent stem
cell compartment (2) but in man it cannot be assayed and inferences
must be drawn from animal experimentation. Briefly the assay for
the pluripotent stem cell or CFU consists of transplanting marrow
or spleen from the animals in question into heavily irradiated syngeneic
mice. The animals are sacrificed after 8-10 days by which time discrete
colonies are formed in the spleen. These colonies arise from a single
cell and morphologically may be erythroid, myeloid, megakaryocytic,
differentiated or 
 Fig. 2: Effect of polycythaemia on the size of the myeloblast-promyelocyte compartment on day 4 after various doses of irradiation. Each point shows the myeloblast-promyelocyte compartment compared with the neutrophil count from a polycythaemic (White square) or normal (black square) mouse. The regression lines were calculated by the method of least squares. In each case the size of the myeloblast-promyelocyte compartment is significantly (P > 0.01) related to the logarithm of the neutrophil count but the regression line for the polycythaemic animals lies significantly (P > 0.01 ) above that for the normal animals. (From Morley et al. 1970, reprinted from Br. J. Haematol. (24)). 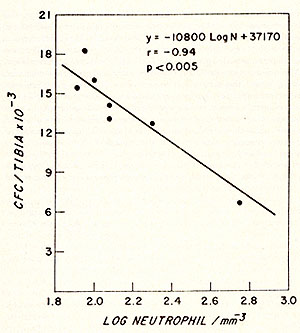
anticipate increased CSF production which would produce the granulocytosis, which is seen under such circumstances. Neutropenia of course is associated wjth infection and increased CSF generation has been reported ( 18) . This hypothesis is appealing but there are still a number of questions to be resolved, principally the problem of tolerance. It is well known that an animal repeatedly injected with endotoxin will become tolerant to its pyrogenic effects and this has been observed in respect to colony stimulating factor (15). Moreover, there appears to be cross-tolerance between various endotoxins. A major problem in assessing the granulopoietic response to avariety ofstimuli has been the storage pool. This has made it difficult to separate granulocytosis due to a true increase in proliferation from release of granulocytes from the storage pool. Further, the possibility exists that the depletion of the storage pool triggers an intramedullary feedback loop which may affect myelopoiesis. The use of the millipore diffusion chamber has been introduced to circumvent this problem (19, 20). Normal bone marrow is implanted into these chambers which are then placed intraperitoneally into host animals. Manipulation of the hosts prior to implantation of the chambers provides an opportunity for the study of growth regulation within the in vivo culture system. Using this technique Tyler and his associate (21) have demonstrated that the proliferation of pluripotent stem cells as well as the growth of myeloid elements is enhanced in neutropenic animals. The best explanation for these results is that a humoral factor is produced in the neutropenic host which diffuses into the chamnbers. The nature of this humoral factor and its relation to the colon y stimulating factor have not been established. From the above We may conclude that rn yelopoiesis is governed by a feedback regulatory mechanism from the peripheral blood and tissues. There is indirect evidence to suggest that this regulation may be accomplished by the glycoprotein, colony stimulating factor, which is responsible for the differentiation of myeloid elements in the soft agar culture system. There is, however, no direct in vivo evidence that CSF plays this central role. Finally the mechanisls governing the production of CSF have not been completely resolved. It is clear that many tissues have the capacity to produce this protein and that endotoxin plays an important role in its generation. Further studies, however, will be necessary before final definitive conclusions can be drawn about its ph ysiologic role in the regulation of mnyelopoiesis.
The two principal types ofmyelocytic leukemia, are the acute leukemmias, including acute myelocytic, progranulocytic and myelomonocytic leukemia, and chronic nnyelocytic leukemia. Patients with chronic myrelocytic leukemia after a variable period of time may enter what is termed a blast cell crisis which in all respects appears to be similar to acute myelocytic leukemia. This is most likely a reflection of the natural history of the disease although the role of chemotherapeutic agents and irradiation in effecting the transformation from CML. to blast cell crisis has not been fully evaluated. It is clear from cytogenetic studies that chronic myeloytic Ieukemia is basically a disease of the pluripotent stem cell compartment. The vast majority of patients have an abnormal chromosome, the Philadelphis chromosome (PHI) which is seen in erythroid and megakaryocytjc cells as well as in the myelocytic; cells. Differentiation in many respects is normal and growth by and large is confined to the bone marrow and spleen, which might be considered a normal hemopoietic organ~ The lack of the enzyme alkaline phosphatase in the mature granulocytes of CML indicates that differentiation is not entirely normal. Further, the intravascular life of the granulocyte in CML is prolonged. Immature cells, myelocytes, promyelocytes and myeloblasts are released into the peripheral blood suggesting some change in the transit pattern from bone marrow to peripheral blood. It has been suggested that the release of granulocytes is normally regulated by membrane properties (22). If so, then the premature release of immature cells suggest a change in the membrane and is further evidence of abnormal differentiation. Although there are these demonstrable abnormalities of the granulocytes, it is clear that in some measure normal regulatory mechanism persists. Oscillation of the peripheral white count and also the platelet count has been documented in a number of patients with CML (23), indicating the existence of a feedback mechanism. In most instances, however, the period of oscillation is longer than normal. The prolonged life span of the granulocytes together with the retention of a measure of feedback control suggests that during its initial phase chronic myelocytic leukemia in part is an accumulative disease. Undoubtedly there is additional input from the stem cell compartment, because arithmetically the prolonged intravascular life span could not account for the level of granulocytosis seen in some patients nor for the oscillation seen in those where oscillatory phenomenon have been documented. The acute myelocytic leukemias may show varying patterns. In some, examination of the bone marrow reveals only abnormal myeloid elements; in others erythropoiesis and megakaryocytopoiesis may be present. Even during the preleukemic phase there may be identifiable morphologic abnormalities in megakaryocytes and erythroid elements. when cytogenetic abnormalities exist, these may be present in the megakaryocytes and erythroid elements as well as the myeloid. These observations suggest that acute myeloblastic leukemia at least in some instances originates from the stem cell. It seems likely to me that this is true for all patients with acute myeloblastic leukemias. Of paramount importance in considering the pathophysiology of acute myelocytic leukemia is the fact that complete remissions can be achieved with chemotherapy and normal hemopoiesis re-established. Thus, normal pluri-potent stem cells must be present in the bone marrow together with the leukemic stem cells. This suggests the existence of clones of pluripotent cells some of which are leukemic the other normal. Thus, if leukemia is of viral origin the abnormal information transmitted by the virus is present in some but not all pluripotent stem cell lines. One might suggest as a corollary that in some instances the viral infection of a stem cell will result in a lethal transformation and death of what might otherwise have become a leukemic clone. In others non-lethal information develops and an abnormal line of differentiation results producing leukemia. In still others the viral infection may not be manifest or affects DNA in such away that normal myelopoiesis is unaffected. The alternative explanation of course would be that some stem cells become infected with virus but others do not. Since the introduction of the soft agar technique for the culture of myeloid precursors there has been considerable interest in a number of laboratories as to the role of the myeloid committed stem cell and the differentiating colony stimulating factor in leukemic patients. It has been reported that normal differentiation may be achieved when bone marrow from aleukemic patient is cultured in vitro. In a rather large survey Moore (24) found that the marrow from about 60% of patients with acute leukemia developed small clusters in culture rather than normal colonies, 20 % developed colonies and 20 % failed to grow. The central issue is whether in cultures of bone marrow from leukemic patients normal colonies develop from a normal pluripotent stem cell or under these cultural conditions are normal myeloid elements differen tiated from a leukemic stem cell line ? Moore and Metcalf ( 25) have reported that cytogenetic markers present in leukemic cells were found in all metaphases of cultured leukemic marrow. Unfortunately pooled rather than single colonies were examined. These data indicate that leukemic cells are proliferating in culture but do not provide the final answer that normal granulocytes develop in culture from leukemic progenitors. To be candid there is relatively little data published on the morphologic aspects of the differentiated colonies from culture of leukemic cells and in a recent conference the photomicrographs which were shown of leukemic cultures did not persuade me that anyone had succeeded in differentiating leukemic cells into normal myeloid elements. It has also been suggested by Mintz and Sachs (26) that an inhibitor may be involved in the failure of leukemic myeloblast to differentiate. Clearly, further information is needed on the characteristics of leukemic cells in the soft agar system. Are normal stem cells differentiating? Are leukemic cells capable of normal differentiation and the production of normal differentiated polymorphonuclear cells? What, if any, is the role of colony stimulating factor in the failure of differentiation? What, if any, is the role of inhibitors in this failure of differentiation ? The concept that myeloid leukemia represents merely a failure of differentiation from the myeloblast stage due to an impaired interaction between colony stimulating factor and the target cells seems to oversimplify the issue. This would be somewhat analogous to pernicious anemia in which the morphologic abnormalities may be as striking as those seen in acute leukemia. This disease has been shown to be the result of abnormal differen tiation due to a deficiency of B 12. Administration of the vitamin immediately corrects the abnormal proliferation. I t seems to me that those who consider acute leukemia in this light overlook one major and important characteristic of the leukemic cell. In pernicious anemia the abnormal cellular proliferation is confined to the normal areas of hemopoiesis -the bone marrow. In acute myelocytic leukemia as in other neoplastic diseases proliferation occurs not only in the site of normal cell production but elsewhere in the body. Thus in acute myelocytic leukemia leukemic proliferation may be seen virtually anywhere, the skin, the heart, the liver, brain, etc. Thus the normal mechanisms which restrict cell production to a specific anatomic area are no longer operative. It seems most likely that the normal mechanism controlling the site of cell production is an interaction between the cells and their immediate environment or microenvironment. Thus in acute myelocytic leukemia the growth pattern reflects not only a failure of differentiation but other cellular abnormalities, most likely in the membrane structure, which permit metastatic growth. In this respect acute myeloblastic leukemia appears to be more than a simple failure of differentiation of the myeloblast. For this reason it seems unlikely that the resolution of acute leukemia will result from efforts directed solely at differentiation of myeloblasts.
1. Stohlman, F., ]r., Quesenberry, P. ]. and Tyler, W.: The Regulation of Myelopoiesis As Approached With In Vivo and In Vitro Technics. Progress In Hematology, in press. 2. Till, ] .E. and McCulloch, E. A. : A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Rad. Res. 14: 213-221,1961. 3. Pluznik, D. H., and Sachs, L. : The cloning of normal "mast" cells in tissue culture. J. Cell. Comp. Physiol. 66: 319-324,1965. 4. Bradley, T. R. and Metcalf, D. : The growth of mouse bone marrow cells in vitro. Aust.J. Exp. BioI. andMed. Sci. 44: 287-300,1966. 5. Chervenick, P. A. and LoBuglio, A. F.: Human blood monocytes: Stimulators of granulocyte and mononuclear colony formation in vitro. Sci. 178: 164-166, 1972. 6. Moore, M. A. S. and Williams, N.: Physical separation of colony stimulating cells from in vitro colony forming cells in hemopoietic tissues. J. Cell. Physiol. 80: 195-206,1972. 7. Gordon, A. S., Handler, E. S., Siegel, C. D., Dornfest, B. S., and Lobue, J. : Plasma factors influencing leukocyte release in rats. Ann. N. Y. Acad. Sci. 113: 766-779, 1964. 8. Boggs, D. R., Chervenick, P. A., Marsh, J. C., Cartwright, G. E., and Wintrabe, M. M. : Neutrophil releasing activity of dogs injected with endotoxin. J. Lab. Clin. Med. 72: 177--185,1968. 9. Athens, J. W .: Neutrophilic grnulocyte kinetics and granulocytopoiesis. In Gordon, A. S. (Ed.): Regulation of Hemopoiesis. New York, Appleton-Century Crofts, 1970. 10. Morley A., King-Smith, E. A., Stohlman, F., J r.: The oscillatory nature of hemopoiesis. In: Stohlman, F., Jr. (Ed.) Hemopoietic Cellular Proliferation. Grune and Stratton, Inc., New York, 1970. 11. Morley, A. A. and Stohlman, F., Jr: Cyclophosphamide induced cyclic neutro penia. New Eng. J. ofMed. 282: 643,1970. 12. Morley, A., Monette, F. C., Rizzoli, V., Howard, D., and Stohlman, F.,Jr.: Studies on the regulation of granulopoiesis III Neutrophil kinetics in irradiation-induced neutropenia. Brit.J. Haemat. 20: 637-642,1971. 13. Rickard, K. A., Morley, A., Howard, D., and Stohlman, F., Jr.: The in vitro colony-forming cell and the response to neutropenia. Blood 37: 6--13,1971. 14. Morley, A., Rickard, K. A., Howard, D. and Stohlman, F., Jr.: The regulation of granulopoiesis IV Possible humoral regulation. Blood 37: 14-22,1971. 15. Quesenberry, P ., Symann, M. and Stohlman, F ., J r. : Unpublished data. 16. Morley, A., Quesenberry, P., Bealmear, P., Stohlman, F., Jr., and Wilson, R.: Serum colony stimulating factor levels in irradiated germfree and conventional CFW mice. Proc. Soc. Exp. BioI. &Med. 140: 478-480,1972. 17. Quesenberry, P. J., Morley, A., Stohlman, F., Jr., and Smith, M.: Effect of endotoxin on granulopoiesis and colony stimulating factor. N. Eng. J. Med. 286: 227-231,1972. 18. Stohlman, F., Jr. and Quesenberry, P. J.: The kinetics of myelopoiesis and their relationship to drug induced neu tropenia. In Dimitrov. N. ( Ed. ) : Hahneman Symposium on Drug Effects on Hemopoiesis. New York, Grune & Stratton, in press,1973. 19. Boyum, A., and Borgstrom, R. : The concentration of granulocytic stem cells in mouse bone marrow, determined with diffusion chamber technique. Scand. J . Haemat. 7: 294-303,1970. 20. Breivik, H., and Benestad, H. B.: Regulation of granulocyte and macrophage formation in diffusion chamber cultures of mouse haemopoietic cells. Exp. Cell. Res. 70: 340-348, 1972. 21. Tyler, W. S., Niskanen, E., Stohlman, F., Jr., Keane, J., and Howard, D.: The effect of neutropenia on myeloid growth and the stem cell in an in vivo culture system. Blood 40: 634-645, 1972. 22. Lichtman, M. A., and Weed, R. I. : Alterations of the cell periphery during maturation. Blood 39: 301-316. 1972. 23. Morley, A. A., Baikie, A. G., Galton, D. A. G.: Cyclic leukocytosis as evidence for retention of normal homeostatic control in chronic granulocytic leukemia. Lancet 2: 1320,1967. 24. Moore, M. A. S.: In Conference OfIn Vitro Culture Technics. W. A. Robinson, (Ed.): in press. 25. Moore, M. A. S. and Metcalf, D.: Cytogenetic analysis ofhuman acute and chronic myeloid leukemic cells cloned in agar culture. Internat. J. of Cancer 11: 143, 1973. 26. Mintz, U. and Sachs, L.: Differences in inducing activity for human bone marrow colonies in normal serum and serum from patients with leukemia. Blood 41: 331, 1973. |