|
* This work was supported in part by grant POICa-20194, awarded by the National Cancer Institute, DHEW, and by the Gar Reichman Foundation. K. Welte is supported by DFG grant We 942/ 1-2 and H. J. Feickert by DFG grant FE 181/1-2 The regulation of immune function [6] and tumor growth [ 16] by hormone-like factors, cytokines, has become the subject of increasing interest. Interleukin 2 (IL2) discovered by Morgan et al. [5], is produced by T -lymphocytes after antigen or mitogen stimulation and is required for the proliferation of activated T cells. IL2 is an essential mediator of the immune response [11, 15], and there is preliminary evidence that it may also be responsible for the clonal growth of human lymphoblastic leukemias [ 17]. Studies on the physiology and pathophysiology of IL2 are dependent on the availability of a well-defined, biochemically, and biological homogeneous molecule. We have therefore purified IL2 to apparent homogeneity [18] and have started to examine its role as mediator of the normal immune response, in human immunodeficiency syndromes and in acute lymphoblastic leukemias (ALL).
Heparinized blood samples were drawn from healthy volunteers and patients after obtaining informed consent. Ficoll-Hypaque separated mononuclear blood cells were resuspended at 4 X 10 high 6 cells/ml in RPMI 1640 supplemented with 5% heat-inactivated FCS and glutamine (2 mM). Each sample was stimulated in triplicate microwell cultures ( # 3596 culture plate, Costar Inc. Cambridge, MA) with one of the following: (a) medium alone, (b) phytohemagglutinin (PRA-M, 0.5% by volume, Grand Island Biological CO), (c) OKT3 (Ortho Diagnostic Systems, Inc., Raritan, NJ) or (d) Pan T2 (Wang et al. 1982, submitted).
I. Physiology of lymphocyte proliferation and IL2 Production Induced by PHA and Mitogenic Antibodies Stimulation assays were done with or without the addition of irradiated Daudi cells (5000 rads) at a final concentration of 0.5 X 10 high 6 cells/ml. At indicated time points lOO µl supernatants were removed from each well to be assayed for IL2. Identical cultures were pulsed for 4 h with tritiated thymidine [³H]dT (0.5 µci/microplate well, specific activity 20 mci/mM. New England Nuclear, Boston, MA) and the incorporation of[³H]dT measured. The IL2 microassay, definition of units, and biochemical techniques have been published in detail elsewhere [I, 18]. 1. Mitogenesis Induced by PHA, Pan T2, and OKT3 PHA as well as both T -cell specific antibodies were able to induce
a proliferative response in normal PBL incubated for 4 days a measured
by incorporation of tritiated thymidine (Fig. 1). There was no significant
difference between the two antibodies when used at saturating concentrations.
However, the amount of Pan T2 (10 high -10 M) needed to induce maximum
mitogenesis was 100 times more than the concentration required for
OKT3 (10 high -12 M). In the presence of irradiated Daudi cells
we observed a twofold increase in cell proliferation with Pan T2.
In contrast, the co stimulation with Daudi cells on OKT3 had essentially
no effect. 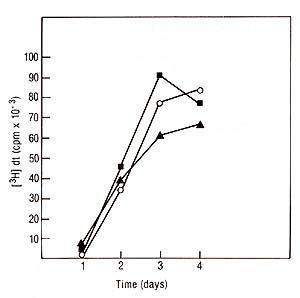
2. IL2 Production Induced by Monoclonal 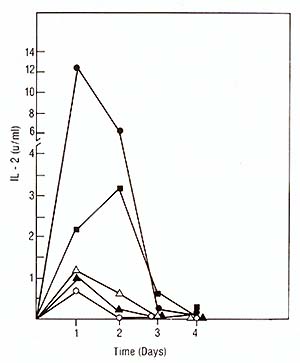
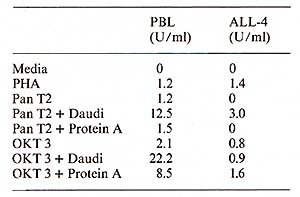
Protein A ( 40 µg/ml) was added to the microwell cell supensions of normal PBL in the presene of either Pan T2 or OKT3. As shown in Table 1, protein A had only a negligible effect on the IL-2 production induced by Pan T2, while it enhanced the stimulation by OKT3 approximately fourfold. (In contrast, irradiated Daudi cells are potent costimulators for the Pan T2 response while they are without effect on the OKT3 response). 4. Inhibition ofI L2 Production and Response Further studies also suggest a role for HLA-DR antigens in the regulation of the IL2 production [7]. The extent of inhibition by antibodies against these structures is dependent on the mitogen used (own observation), suggesting that Pan T2 and OKT3 bind to different subunits of the T -cell activation antigen recognition complex. This has recently been continued through immunoprecipitation studies (Wang et al. 1982, submitted). 5. Stimulation of P EL Proliferation by Anti M7andAnti RD 114Antiserum Table 2 shows that goat antisera raised against the baboon endogenous
virus, M7, or RD114 were able to stimulate the proliferation of
PBL, while goat anti-simian sarcoma virus (SSV) antiserum was unable
to do so. Absorption of the antisera with M7 or RDl14 virus removed
the sera capacity to induce cell proliferation. These data suggest
a common antigenic determinant shared by the T -cell activation/
antigen recognition complex and M7 as well as RD 114.
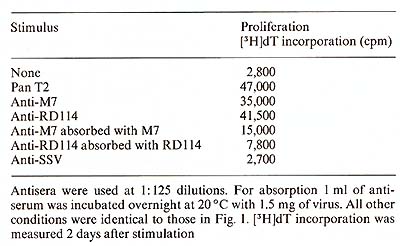 Table 3. Biochemical characteristics of IL2 produced by PBL and leukemic lymphoblasts (ALL) in the presence and absence of Daudi cells 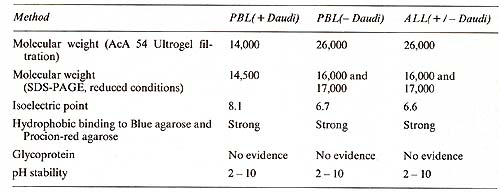 II. Biochemistry The purification of IL-2 from lymphocyteconditioned medi urn (L y-CM) has been reported in detail elsewhere [18]. Briefly, IL2, produced with or without costimulation by irradiated cells of the Burkitt's lymphoma line Daudi, was purified 37,000-fold to apparent homogeneity from Ly-CM by sequential (NH4)2S04-precipitation, ion exchange chromatography (DEAE-cellulose), gel filtration, and chromatography onblue agarose and on Procion-red agarose. The purified IL2 showed a specific activity of 10 high 6 U/mg protein. IL2 produced in the absence of Daudi cells exhibited a native molecular weight of 26,000 as measured by gel filtration and an isoelectric point of 6.7. This IL-2 showed 16,000 and 17,000 mol wt. bands in SDS-polyacryladmide gel electrophoresis. IL-2, produced in the presence of Daudi cells, showed a molecular weight of 14,000, as measured by both gel filtration and SDS-polyacrylamide gel electrophoresis, and an isoelectric point of 8.1 (Table 3). The purified IL-2 lacked detectable activities of all cytokines tested: interferon (alfa and epsilon), granulocyte-macrophage-colony stimulating factor, E-cell growth factor, T -cell replacing factor, E-cell differen tia tion factor, macrophage acti vation factor, and thymocyte-differentiating activity. It was free of any contaminating proteins as judged by silver staining in SDS-polyacrylamide gel electrophoresis. All three molecular forms of IL-2 were biologically active, supporting the growth of human and murine cytotoxic T -cell lines at concentrations of 10 high -11 - 10 high-10 M.
We used the purified IL2 for the production of a mouse monoclonal antibody against IL2. The fusion resulted in hybrid clones producing anti-IL2 of various subclasses (IgA, IgG-2b, IgM). All antiIL2 antibodies inhibited the proliferation of IL2-dependent human and mouse cell lines in reponse to human highly purified IL2. One of these antibodies chosen for further characterization precipitated 14K 125I-IL2 as well as 16K125I-IL2 and 17K 125I-IL2 (Feickert et al. 1982, submitted). IV. IL2 Production by Fresh Lymphoblastic Cells and the Lymphoblastic Cell Line JM * * The cell line Jurkat used by other investigators is a subclone of the originalline JM developed by Schneider et al. [13]. Our studies have failed to show any difference between the original JM and the subclone Jurkat and therefore consider the original designation JM more appropriate 1. Production oilL2 by Leukemic Cells Leukemic cells were cultured in the presence of PHA, OKT3, or Pan T2, and tested for IL-2 production and proliferation as described for PEL. IL-2 production induced by PHA and OKT3 stimulation continued to increase over 3 days (Fig. 3) and was not followed by a rise in cell proliferation (not shown). This was in marked contrast to the reponse of PEL to PHA and OKT3 stimulation (Fig. 2). This pattern of response was common to ALL with different phenotypes. Pan T2 was unable to induce either proliferation or IL2 production in ALL. This pattern of response was also markedly different to Pan T2 stimulation of normal PEL. JM, a cell line derived from a T -cell ALL, is TdT+, la-, E+, Leu I+ and, after PHA stimulation, produces IL2 but does not proliferate. We studied the effect of Pan T2 on this line and found that this monoclonal antibody does not induce IL2 production or stimulate cell proliferation. Therefore, JM and fresh ALL cells have the same pattern of IL2 production and proliferation after PHA or Pan T2 stimulation (Table 1). The addition of Daudi cells was able to rescue the response of ALL cells to Pan T2, and induced IL2 production. We could not detect any effect of Daudi alone on IL2 production by any of the ALL cells (Table 1) and JM (not shown). 2. The Factor Produced by the Leukemic Population is IL2 In order to show that the factor produced by the leukemic cell
populations was indeed IL2, we tested if the factor produced by
ALL cells was able to support the growth of the human cytotoxic
cell line, C13.3 (kindly provided by Dr. N. Flomenberg, Sloan-Kettering
Institute), which requires IL2 for survival and proliferation. The
factor produced by ALL and IL2 puri fied from normal PEL supported
the growth ofC13.3 equally well. As shown in Table 3 the biochemical
characteristics of IL2 produced by leukemic cells are similar to
those of IL2 produced by normal PEL. However, the molecular heterogeneity
of IL2 produced by leukemic cells was not influenced by Daudi costimulation
in contrast to IL2 generated by normal PEL. Finally, IL2 produced
by ALL cells binds to a monoclonal antibody prepared against rat
IL2 (analysis performed by Dr. Gillis, Immunex, Seattle, W A) as
well as to our own monoclonal antibody against human IL2 (Welte
et al., unpublished). A colony assay for blast cell progenitors
in non-E non- TALL has recently been described by Izzaguire et al.
[3]. ALL cells were cultured in methylcellulose in the presence
of Ly-CM and feeder T cells. After 5- 7 days the colonies exhibited
the common ALL phenotype. To test whether growth was dependent on
IL2 we substituted partially purified IL2 (DEAE-cellulose fraction)
and purified IL2 for the Ly-CM in the presence and absence of feeder
T cells. Preliminary results suggest that partially purified IL2
alone can support the growth of ALL cells; however the highly purified
IL2 reuires feeder T cells for maximum colony formation (Table 4).
The cell surface markers of the colonies grown in the presence of
purified IL2 exhibited both pre-B and T -cell characteristics. In
a more recent experiment with another ALL donor, the majority of
the cells were Bl-positive. We are currently testing several additional
ALL samples in this assay, and will identify the growth factor requirements
of ALL cells in culture to investigate further the hypothesis of
autostimulation in ALL. 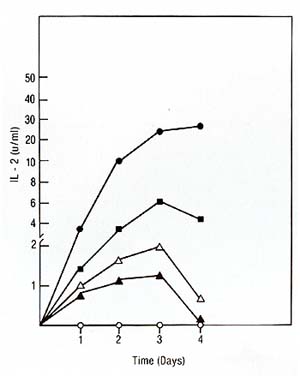
1. Mouse model Spleen cells from mice treated with cyclophosphamide (CY) (150 mg/kg) do not generate effective cytotoxic T -lymphocyte (CTL) responses to allogeneic tumor cells in vitro. When purified human IL2 is added to the culture system, spleen cells from CY -treated mice are able to generate normal CTL responses (Merluzzi et al. 1982, submitted). 2. Combined Varied Immunodeficiency (CVI) Fifteen patients with CVI and one patient with a related disorder (transient hypogammaglobulinemia of childhood) had a statistically significant decreased response to mitogen stimulation when compared with a control normal population. After addition of purified IL2 the proliferative response was significan tly im proved with all mitogens used. Two groups could be distinguished: Group A (10/16) had full or partial normalization of proliferative response after addition of IL2, and group B (6/16) had no significant response. One patient showed a decrement in proliferative response after IL2 was added. The results are listed in Table 5 and in Kruger et al. ( 1982, manuscript submitted). The production of endogenous IL2 was lower in the group of patients irrespective of the mitogen used when compared with normals (Table 5). The anti- T -cell monoclonal antibody, Pan T2, recognized a proliferative defect in 5/16 patients which was neither recognized by PHA nor OKT3. This was not significantly correctecd by the addition of IL2. The lack of responsiveness to Pan T2, however, did correlate with the inability of the E cells of these patients to proliferate and differentiate in reponse to B-cell mitogens [ 12]. Table 4. Growth factor requirements of
ALL cells in methylcellulose culture 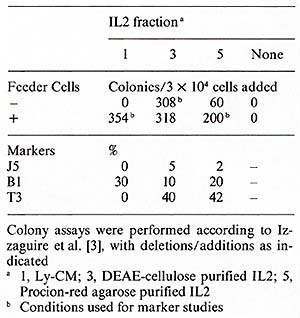
3. Kaposi's Sarcoma in A cquired Immunodeficiency Syndromes (AID) Homosexual patients with Kaposi's sarcoma (KS) exhibited a very low proliferative response to OKT3 ( 15% of the normals). Four out of seven patients also had a very low proliferative response to PHA (10% of the normals) and to Pan T2 (8% of the normals). The production of endogenous IL2 was significantly lower in PEL cultures from KS patients, irrespective of the mitogen used. Addition of purified IL2 in the presence of these mitogens was able to restore partially or completely the lymphocyte proliferation in all patients tested. These data suggest that homosexual patients with KS have a defect in IL2 production that is correctable, in vitro, by addition of purified IL2 (Ciobanu et al. 1982, submitted) (Table 5). Table 5. IL2 production and proliferation
(in the absence and presence of 10 U /m1 purified IL2) of PBL from 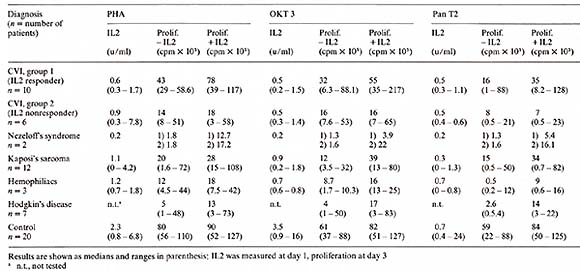
All patients with relapsed Hodgkin's disease before retreatment showed a decreased T -cell proliferative response to all mitogens used and had a partial normalization of T -cell proliferative response after addition of purified 112 (Table 5). 5. Hemophiliacs with Acquired Immunodeficiency Three of five patients with hemophilia examined had an abnormal T -cell proliferation pattern purified with a partial normalization in the presence of IL2 (Bussel et al. manuscript in preparation) (Table 5). 6. Primary Immunodeficiency Syndromes One child with Nezelofs syndrome showed in vitro restoration by purified IL2 of the proliferation in reponse to alloantigens and mitogens. After 6-day MLC in the presence of IL2, effector cells capable of NK and alloreactive cytotoxicity against PHA Iymphoblasts and neoplastic cell lines were recovered. No viable cells were recovered from similar in vitro cultures in the absence of IL2. A second N ezelofs patient showed augmentation ofhis NK activity but no restoration of the allocytotoxic reponse. The proliferative response to PHA, OKT3, and Pan T2 is shown in Table 5. Patient 1, who demonstrated a positive in vitro response to IL2, was subsequently given IL2 subcutaneously as part of a recently initiated phase I trial. Though the patient died several days after the trial was begun due to pulmonary infection, examination of his lymphoid tissues postmortem suggested that purified IL2 may have exerted an in vivo effect on his T -lymphocytes. At autopsy, his lymphoid tissues showed only histocytes and plasma cells except in the lymph nodes draining the IL2 administration sites, where nests of lymphoid cells were identified. These studies suggest that some primary and acquired immunodeficiencies may be caused by defects in IL2 production and/or response. In addition, they have provided some preliminary evidence that highly purified IL2 is capable of producing an in vivo effect in appropriate immunodeficient patients (Flomenberg et al. 1982, submitted).
I. Physiology of IL2 Production and Response The introduction of T -lymphocyte specific monoclonal antibodies
has facilitated the ability to comprehend further the complex interaction
and control of the immune response. The binding of the antibodies
Pan T2 and OKT3 to specific antigenic determinants (T -cell activation/antigen
recognition complex) on the surface of T -lymphocytes is able to
trigger a proliferative response similar to antigenic stimulation
or mitogensis. It has been observed that OKT3 is mitogenic even
in the range of 10 high -12 M, while Pan T2 is less potent, requiring
a concentration of 10 high-10 M for maximum stimulation. While highly
costimulatory with Pan T2, Daudi cells had no significant effect
on either IL2 production or cell proliferation in the presence of
OKT3. Daudi cells have been used by several investigators to enhance
IL2 production from normal PBL [2, 10, II, 18]. The effect of Daudi
cells could be mediated by (a) la antigen, (b) Fc receptors, and
(c) an additional effector molecule. Both la antigen [7] and Fc
receptors [14] have been implicated in the augmentation of IL2 production.
II. Biochemistry The purification steps described in this study produced IL2 with a specific activity of 10 high 6 U /mg protein. Because the lowest molecular weight of an active IL2 polypeptide was 14,000, it could be calculated that IU/ml of IL2 was equivalent to a molar concentration of 7x 10 high-11 M. An IL2 concentration of 1.4 X 10 high-11 M, or 4 X 10 high 5 molecules/cell, was required for one-half maximum stimulation of murine CTLL. All other purification methods [2, 4] have achieved neither a specific activity nor a yield comparable to those described here. Native IL2 has been previously shown to exist in several molecular forms. Here, we show that the stimuli used for IL2 induction by PEL can be responsible for this heterogeneity. IL2 produced in the presence or absence of Daudi cells had a molecular weight of 14,000 and 26,000, respectively, by gel filtration and 14,500 and 16,000-17,000 repsectively by SDS-polyacrylamide electrophoresis. All molecular forms could be obtained by varying the concentration of costimulator cells. The effect of Daudi cells on the IL2 production, however, does appear to be complicated in view of(a) the shift in molecular weight of IL2 induced by Daudi cells in PEL, and (b) the superinduction of IL2 in PEL and in human lymphoblastic leukemic cells by costimulation with Daudi cells. The possibility that different T -cell subsets or different leukemic phenotypes are responsible for the production of the two IL2 forms is currently under investigation.
There is evidence that the growth of at least some human malignant
cells is factor dependent and that the malignant cells are capable
of producing these factors ("autostimulation," [16]). We have studied
the capacity of leukemic cells to produce and respond to IL2. The
leukemic cells studied were either nonT, non-E ALL, or T -cell ALL.
In every case, the cells produced a large quantity of IL2. This
factor had physicochemical characteristics identical to that of
normal IL2, with a mol. wl. of 16,000-17,000 and pI of 6.6 (Table
3) and reacted with monoclonal antibodies directed against normal
IL2. These data therefore strongly support that the factor produced
by ALL cells is identical or at least closely related to IL2. Costimulation
of ALL cells by PHA and Daudi cell, however, failed to lead to a
shift in molecular weight, suggesting a restricted expression of
IL2 species in ALL. Further studies performed argue against the
possibility that residual normal T cells are responsible for the
IL2 production by extensive cell purification techniques (repeated
E-rosetting, density gradient centrifugation of hypodiploid or hyperdiploid
leukemic cells). The leukemic population studied could not have
had more than 1% normal cells based on flowcytometric analysis of
DNA ploidy levels. While OKT3 was able to induce IL2 production
from leukemic cells, Pan T2 alone was unable to cause the release
of IL2 from ALL cells. However, the Pan T2 activation "pathway"
is not completely repressed in ALL cells since it can be activated
by costimulating ALL cells with Pan T2 and Daudi. The pattern of
response of ALL cells to Pan T2 and Daudi suggests that the IL2
producer cell in the ALL population has an altered Pan T2 receptor
complex, which could playa role in leukemogenesis. This conclusion
is supported by the study of JM, aleukemic T -cell line. The characteristics
of IL2 production in this clonal population of leukemic cells were
found to be similar to those of ALL cells. How Daudi cells are able
to restore the ability of Pan T2 to induce IL2 may be important
in further understanding the lack of normal control mechanisms on
cell proliferation in ALL. The role of IL2 production in ALL remains
to be determined. It appears unlikely that the release of this factor,
critical for the proliferation of cells of T lineage, is only an
epiphenomenon in ALL, irrelevant for the expansion of the leukemic
clone. Recently, a clonal assay system permitting the growth of
blast cell progenitors in non- T, non-E ALL has been developed [3].
In this assay, factors present in Ly-CM are required for the successful
growth of the leukemic stem cells. Since (a) IL2 is present in Ly-CM,
(b ) 1L2 produced by leukemic and normal T cells appears to be identical
(Table 3), ( c) peripheral blast populations of ALL do not proliferate
in response to IL2, as measured by [³H]dT incorporation, we hypothesize
that IL2 is a factor ( or one of the factors) produced by partially
differentiated leukemic cells and required for the replication of
the leukemic stem cells. Since leukemic stem cells represent only
a small percentage of the total leukemic population, its proliferation
cannot be shown in the assays used for normal PEL. The clonal assay
should be able to clarify this important point. Our preliminary
data suggest that highly purified IL2 is able to substitute for
Ly-CM (Feldman, Izzaguire, Mertelsmann, unpublished data). 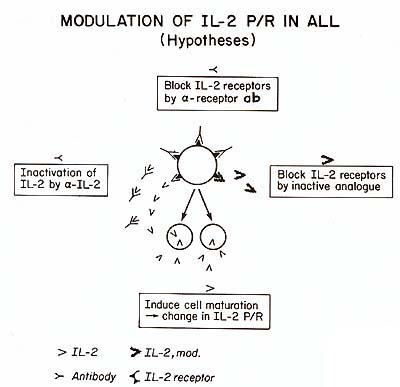
IV. IL2 in Immunodeficiency Syndromes It is well known that IL2 plays an important role in the development of a variety of T -cell responses. We suggest that some human disorders associated with T -cell defects might be due to defective IL2 production or response. We have recently begun to investigate the role of IL2 in primary and acquired immunodeficiency syndromes. The data obtained so far show complete or partial normalization of T -cell proliferation by purified ILl in vitro in the majority of patients with Kaposi sarcoma, CVI, Hodgkin's disease, hemophiliacs with acquired IDS, chemotherapy-induced immunosupression (data not shown), and burn patients ( data not shown). Since these observations suggest an important in vivo role of ILl in several congenital and acquired IDS, we have initiated a phase I clinical trial of ILl. The preliminary results support in vivo activity of subcutaneously administered ILl, both in animal models (Merluzzi et al., unpublished) and in man (Flomenberg et al., unpublished).
We would like to thank Ms. Maureen Sullivan and Ms. Lorna Bamett for their technical support and Ms. Cynthia Garcia for the typing of the manuscript.
I. Gillis S, Ferm M, au w, Smith KA (1978) T cell growth factor:
Parameters of production and a quantitative microassay for activity.
J Immunol120: 2027 |