|
A. Introduction One of the mechanisms capable of regulating an immune response
involves the interaction of idiotypes and anti-idiotypes. In selected
nontumor systems it has been shown that anti-idiotypic immunity
can very specifically either suppress or stimulate immune responses
(for review see Eichmann 1978). Thus, antiidiotypic reagents may
provide powerful tools for manipulating the induction or course
of the host's immune responses to malignant cells. To test this
hypothesis, we have developed a tumor model in which anti-idiotypic
immuni ty of mice to syngeneic tumor-specific lymphocytes can be
elicited and analyzed (Flood et al. 1980). It had previously been
shown that animals immunized with syngeneic purified antigen-specific
lymphoblasts isolated from mixed lymphocyte cultures can produce
antiidiotypic immune responses to their own alloantigen reactive
T cells (Andersson et al. 1976, 1977; Aguet et al. 1978; Binz and
Wigzell 1978). We have adapted this system to syngeneic responses
against tumor antigens and we can now manipulate the immune response
in such a way that tumors will grow in animals which would normally
reject these tumors.
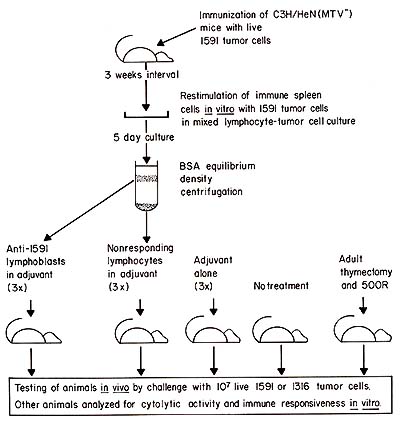
Fig. I. Experimental protocol used for the generation and purification of tumor-specific Iymphoblasts, immunization with these cells, and subsequent testing of blast-immunized and control animals. For details see Flood et al. ( 1980 )
The fibrosarcomas 1591 and 1316 induced by ultraviolet light in
C3H/HeN (MTV-) specific pathogen free mice were used (Kripke 1977).
These tumors have non-cross-reactive tumor antigen and regularly
regress upon transplantation into normal young syngeneic C3H mice
(Fisher and Kripke 1977), but grow progressively in thymectomized
x-irradiated mice. The general approach for induction of anti-idiotypic
immunity by immunization with tumor-specific lymphocytes is shown
in Fig. 1 and has previously been described in detail (Flood et
al. 1980). 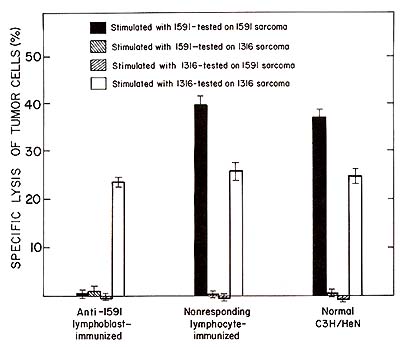 Fig. 2. Specific unresponsiveness of spleen cells from anti-1591 Iymphoblast-immunized animals to 1591 tumor cells in culture. Spleen cells were stimulated in a 5 day primary mixed lymphocyte tumor cell culture with 1591 or 1316 tumor cells. The culture-generated effector cells were tested in a 3 h 51Cr-release assay at a 100 : 1 effector to target cell ratio. Blast-immunized or control animals were tested 10 days after the third immunization
We have found that immunization with tumorspecific lymphoblast induces unresponsiveness to 1591 tumor cells in vivo and in vitro (Fig. 2). Furthermore, we have found that immunization with 1591 specific lymphoblasts induced cytolytic anti-idiotypic T cells to these lymphoblasts. This was shown by an in vitro assay in which 1591 tumor-specific lymphoblasts were used as 51-Cr-labeled target cells for the anti-idiotypic effector cells. Several lines of evidence suggested the specificity of the observed effects. Resistance of the 1591 blast-immunized animals to 1316 tumor cells in vivo and responsiveness of the spleen cells from these animals in vitro were unimpaired. In agreement with this is the finding that spleen cells from the 1591 tumor-specific lymphoblast-immunized animals did not kill 1316 tumor-specific lymphoblasts. Furthermore, immunization with nonresponding lymphocytes or with lymphoblasts not having specificity for 1591 tumor cells were ineffective in inducing the observed effects. Strong evidence indicating idiotype-specific immune reactions to tumor-specific lymphocytes came from the analysis of animals which responded to the tumor in spite of the blast immunization. These animals exhibited the same capability to specifically lyse 1591 tumor cells as control animals, yet they responded by generating 1591 specific lymphoblasts that were completely insensitive to lysis by antiidiotypic effector cells (Table 1). This is in contrast to normal and control animals, which regularly responded by generating tumor-specific lymphocytes sensitive to the anti-idiotypic effector cells. Table 1. Presence of a common 1591 specific
idiotype on tumor-specific lymphocytes from normal and control animals
which is absent in the tumor-specific lymphocytes of animals which
broke idiotype-specific Immune suppression 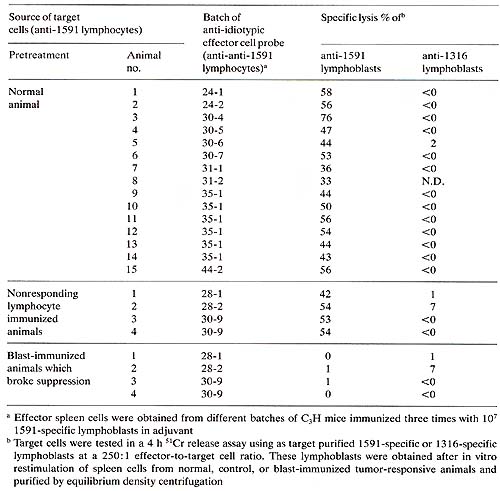
The findings suggest that autologous idiotypespecific immunity against a tumor-specific lymphocyte clone, which is regularly present in normal animals (Table 1), can be induced by the blast immunization. Thus, immunity eliminated the animals' normally predominant tumor-reactive lymphocyte clone, and these animals died of progressive tumor growth when challenged with the 1591 tumor cells, unless they were capable of breaking suppression with an idiotypically different, previously silent or undetected lymphocyte clone. The possible role of antigenic stimulation in the development of new clones ( Cunningham and Pilarski 1974) is not clear in our system, but we have only observed the development of secondary 1591 specific clones in animals which have previously been blast immunized. Our experiments give strong evidence for the importance of tumor-specific lymphocytes in the primary defense of the onimmune host to malignant cells and raise the possibility that natural killer cells may not playa decisive role in the defense of C3H mice against these tumors. Interestingly, older mice or ultraviolet light irradiated young mice which are susceptible to the 1591 and 1316 tumors cannot develop 1591 specific lymphocytes in vivo or in vitro, while they do develop natural killer activity (manuscript in preparation). It will be interesting to determine whether anti-idioty pic reagents, used under defined conditions, may stimulate tumor-specific lymphocyte clones in such animals and thereby induce in these mice resistance to the malignant cells.
This research was supported by USPHS Grants R01-CA-22677 and R01-Ca 27326 to H. Sand CO- 75380 to M. L. K; H. S. is supported by RCDA CA-00432, P. F. by NRSA T32-Al- 7090, and J. U. by T32-GMS- 7281.
- Aguet M, Andersson LC, Andersson R, Wight E, Binz H, Wigzell
H (1978) J Exp Med 147:50-62 |