|
A. Introduction Since type-C retroviruses are known to be involved in naturally
occurring leukemias of many animal species [26], a similar viral
etiology has been sought in human leukemias. Some of the animal
models provide important insight for consideration of human leukemias.
For example, while most virus-induced animal leukemias and lymphomas
are associated with abundant virus production in the tumor cells,
bovine leukemia virus (EL V), the causative agent of bovine leukemias,
was not detected until the leukemic cells were cultured in vitro
(see review by Miller and Van Der Maaten [13]). This brings out
the importance of long-term culture of the appropriate target cells
for virus detection and isolation. In 1976, our laboratory reported
the discovery of a factor termed T-cell growth factor (TCGF) [ 15].
Following interaction with an antigen, different subsets of mature
T cells respond by making and releasing TCG F or making a receptor
to TCG F. The TCG F binds to the receptor-bearing T cells and induces
cell growth. Addition of exogenous TCGF can maintain growth of activated
mature T cells for long periods [6, 26]. When TCGF was added to
T cells obtained from patients with mature T -cell leukemias and
lymphomas, some cells directly responded without prior activation
in vitro [18]. Some of those samples released a retrovirus which
we call human T -cell leukemia-Iymphoma virus (HTL V) ([19, 20];
Popovic et al., in preparation). The morphology of HTL V is typically
type c. Figure 1 shows an electron micrograph of some HTL V particles.
Like all retroviruses, HTL V contains reverse transcriptase, has
a high molecular weight RNA genome, and buds from cell membranes.
It is distinct from all other known animal retroviruses [9, 16,
22, 23] and to date is the only unequivocal human retrovirus. (The
retrovirus later isolated independently in Japan [14, 31] and called
ATLV is, in fact, HTL V.) Furthermore, it is specifically associated
with certain forms of human leukemia and lymphoma [4]. Here we wish
to describe some of the new isolates of HTL V and report on some
recent findings on the nature and distribution of HTL V and its
transmission to and biological effects on normal T -lymphocytes
in vitro. Cell lines derived from patients with leukemia/lymphomas
of mature T cells from geographically different parts of the world
have been established in culture in the presence of TCGF as described
previously for normal mature human T cells [ 15, 26] and neoplastic
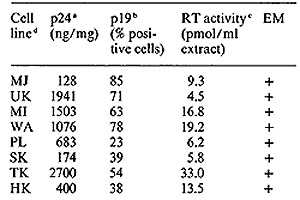
mature T cells [ 18 ]. These cell lines were analyzed for HTL V
by (a) competition radioimmunoprecipitation assay (RIPA) for the
major core protein p24 [9], (b) indirect immune fluorescence assay
(IF A) using highly specific monoclonal antibody for another HTLV
antigen, pl9 [24], (c) reverse transcriptase activity in the culture
fluids, and (d) electron microscopy. In addition to the positive
cell lines CR [19] and MB [20] published earlier, seven of eight
recently established T -cell lines fully expressed HTL V (Table
I) and one showed partial expression, These patients include four
individuals from the United States, one from Israel, and three from
a single Japanese family from the northwest part of the Honshu Island
in Japan. In this family, the patient SK with acute T -cell lymphoma
(A TL) and both his parents are virus positive. The father (MK)
is clinically healthy and the mother has persistent lymphocytosis,
which is considered to be a preleukemic state. All cell lines have
karyotype and HLA patterns that match those of the donors, and the
HLA profiles are different for all eight cell lines. In addition,
a T -cell line established by Golde and colleagues from a patient
(MO) with hairy cell leukemia [27] was positive vor HTL V p 19 and
p24 [II]. We propose to designate each virus isolate with a subscript
of the patient's initials, e.g., HTL V CR, HTL V MB, etc. All isolates
except HTL V MO are highly related to each other as assayed by competitive
radioimmunoassay with p24 (Fig. 2) and hybridization of viral cDNA
to mRNA of the producer cell lines (Fig. 3). By these assays, the
virus of Japanese A TL is indistinguishable from the prototype HTL
V as exemplified by the earlier isolates HTL V CR and HTL V MB.
On the other hand, HTL V MO competes poorly in the p24 assay (Fig.2),
and nucleic acid sequence homology with HTL V CR was detected only
under very nonstringent hybridization conditions ( our unpublished
data). Therefore, this virus may form a distinct subgroup in the
HTLV family. We propose to group them as HTLV-IcR, etc. versus HTLV-IIMo.
a. Detected by competitive radioimmunoprecipitation
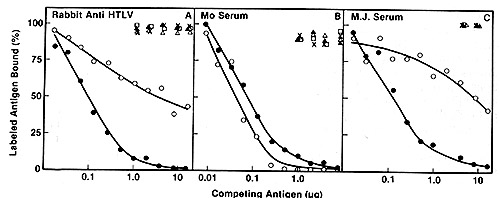 Fig.2A-C. Homologous and heterologous competition radioimmunoassays of HTL v p24. Assays were carried out as described [9] using l25I-labeled HTL V CR p24 and a limiting dilution of hyperimmune rabbit antibody to HTLV CR or sera from patients MO and MJ. A Competition RIA using rabbit anti-HTL V CR. B Competition RIA using MO serum. C Competition RIA using MJ serum. virus extracts used for competition were: black circle - black circle, HTLV CR; white circle- white circle , HTLV MO; x-x, Mason Pfizer monkey virus; white triangle- white triangle, bovine leukemia virus; white square - white square , Rauscher murine leukemia: black triangle - black triangle, simian sarcoma virus 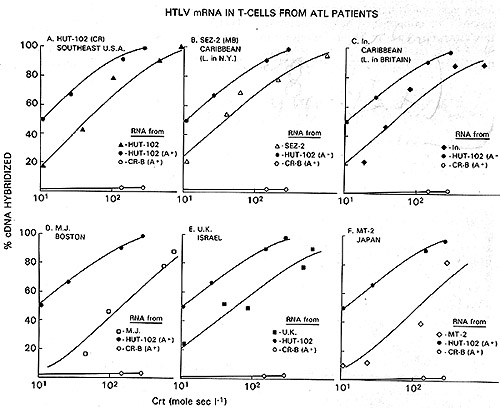 Fig.3A-F. Relationship of different HTL V isolates by RNA hybridization. ³H-HTL V (R cDNA was synthesized by calf thymus DNA primer [22] and hybridized to cellular RNA from different HTLVpositive cell lines. Results with the controls with poly(A) + RNA from CR- T (HUT 102) and CR-B cells are superimposed on each panel for comparison with the others 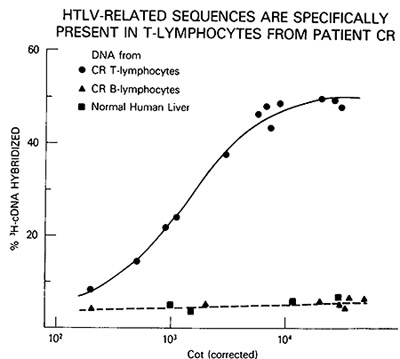 Fig.4. HTL V proviral DNA in T- but not B-celllines of patient CR, ³H-HTL V cDNA was annealed to cellular DNA from CR-T lymphocytes (black circle), CR-B lymphocytes (black triangle), and PHA-stimulated normal human peripheral blood T -lymphocytes (black square) C. HTLV Provirus in Neoplastic T Cells: Evidence for Exogenous Infection We had reported earlier that HTL V sequences are present in the
infected cells and not in normal uninfected human cells [22], suggesting
that HTL V is not an endogenous human virus. In the case of the
patient CR, we also had the opportunity to find out whether he was
infected pre- or post-zygotically [5]. Several T -cell lines, some
clonal derivatives of these lines and a B-cellline have been established
from CR. These cells were shown to have originated from the same
individual by HLA typing. HTL V proviral DNA was detected in some
but not all of the independently established T -cell lines of CR
and not in the B cells. An example of the DNA hybridization kinetics
is shown in Fig. 4. Furthermore, the surface phenotype OKT3-, OKT4+,
and OKT8appears to correlate with the presence of HTL V. These results
indicate that HTL V was acquired by CR by horizontal transmission
and suggest that only a subtype of T cells is the target for HTL
V infection. D. Clinical Features of HTL V -Positive Diseases Seroepidemiological studies have identified HTL V -positive patients
from many regions of the world with at least three major areas that
appear to be endemic: Southwestern Japan [4, 8, 9,25], the Caribbean
[I], centraI South America (see also Blattner et al., this volume),
but only sporadically in the United States [21 ]. Similar clinical
features are found in the diseases associated with these areas,
i.e., Japanese adult T -cellleukemia (ATL) and T-cell lymphosarcoma
cell leukemia (T- LCL ) in the West Indian Blacks from the Caribbean.
Both are represented by an aggressive course an frequent association
with lymphadenopathy, hypercalcemia, hepatosplenomegaly, and cutaneous
manifestations [2, 28]. The tumor cells are all mature, lack terminal
deoxynucleotidyl transferase and express differentiated functions.
Typing with monoclonal antibodies as well as functional studies
showed that the cells may be either of the helper-inducer or suppressor-cytotoxic
phenotype. Histologically, the cells are pleomorphic, often with
highly convoluted nuclei. Almost all patients with A TL and T-LCL
are HTLV positive. These observations led to the hypothesis that
HTL V is associated with a subtype of adult T -cell malignancy which
may include an aggressive form of cutaneous T -cell lymphoma (CTCL)
found in patients CR and MB. In fact, the presence of HTL V may
be of practical importance in disease classification. However, at
least two HTL V -positive patients have relatively benign diseases:
MJ with Sezary syndrome and MO with T -cell hairy cell leukemia.
It should be noted, however, that at least the virus in MO is significantly
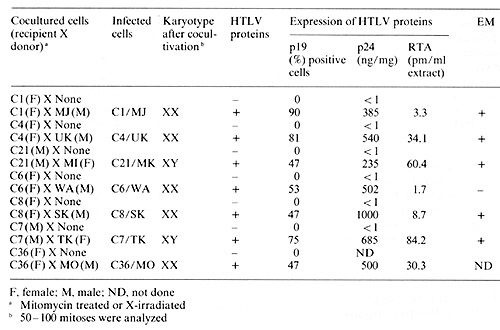
different from the prototype HTL V. E. Infection and Transformation of Human Cord Blood T Cells by HTL V In Vitro Seven of the HTL V isolates described above have been successfully
transmitted into fresh human cord blood T cells by cocultivation
(Popovic et al., in preparation). The virus-positive neoplasic cells
used as donors were first treated with mitomycin-C or X-irradiation
before cocultivation with recipient cord blood cells. After 4 weeks,
assays for T -cell markers, HTL V, karyotype, and HLA-typing were
performed. As shown in Table 2, all recipient cord blood are mature
T cells, positive for HTL V provirus, and express various levels
of HTL V antigens (pI9, p24, and RT). Karyotype and HLA typing consistently
matched the recipient cells. Since cord blood T cells from the same
donors were consistently negative for HTL V markers and the plasma
from their cord blood were also negative for HTL V antibodies, we
conclude that the virus was transmitted from HTL V -producing neoplastic
T -cell lines into cord blood recipient T cells. F. Possible Molecular Mechanism of Transformation by HTL V As mentioned earlier, analysis of HTL V positive leukemic T cells showed that the cells are of clonal origin with respect to the provirus integration sites. In animal systems monoclonality has also been shown to be a common feature of leukemias induced by retroviruses which are chronic leukemia viruses but not those induced by retroviruses which are acute leukemia viruses. Consequently, in spite of its high efficiency to transform T cells in vitro, HTL V probably does not carryon onc gene. Several chronic leukemia viruses are known to induce leukemia by activating cellular onc genes (myc in B-cell lymphomas and erb in erythroleukemias) ([7]; Kung, personal communication) by integrating in the proximity of these genes. Activation of thcse genes is brought about by providing either a viral promotor or viral nucleotide sequences dubbed ""enhancer" [12, 17], the real function of which is still unknown. Since HTL V specifically transforms mature T cells, it is likely to affect expression of genes that are important in T -cell proliferation. A model has been proposed for the mechanism of leukemogenesis by HTL V [4]. Briefly, the HTL V envelope protein interacts with the population ofT cells normally designed to make TCGF receptors, mimicking an antigen stimulation of blastogenesis. These cells then synthesize receptors for TCGF. Simultaneously, the HTLV provirus integrates in the vicinity of the TCGF gene or a gene that exerts a pleiotropic effect on TCGF expression and activates this gene either hy direct promotion or enhancement. The production ofTCGF by a cell bearing a TCGF receptor may result in autostimulation and increased cell proliferation. As an approach to study the gene(s) activated by HTL V infection, we have recently identified and isolated a gene that is expressed at high levels in all HTL V -positive neoplastic T cells and in normal cord blood T cells after infection with HTLV hut not the uninfected counterparts [12 b]. Study of the expression pattern of this gene in uninfcctcd human hematopoietic cells suggests that its expression may be linked to TCGF production. Experiments are in progress to determine if HTL V integrates at a preferred locus in the human chromosome and affects transcription of specific cellular genes in the vicinity, including this gene in question. References 1. Blattner WA, Kalyanaraman VS, RobertGuroff M, Lister TA, Galton
DAG, Sarin PS, Crawford MH, Catovsky D, Greaves M, Gallo RC (1982)
The human type-C retrovirus, HTL V, in Blacks from the Caribbean
region, and relationship to adult T -cell leukemia/lymphoma. Int
J Cancer 30:225-264 Read more
|