|
for ALL Brazilian Group. State University of Campinas, sao Paulo, Brazil.
The Brazilian Cooperative Group for Treatment of Childhood Acute Lymphocytic Leukemia (GBTLI) has started clinical activities trials in 1980.Three consecutive multicenter studies in children with unprevious treated ALL have been completed including 994 patients. The first GBTLI80 accrued 203 children from 1980 to 1982. It was delineated with the standard three drugs induction therapy, CNS protection for all pts comprised cranial irradiation and intrathecal Methotrexate. For low risk pts cranial irradiation with 18Gy was compared in a randomized trial with 24Gy. Maintenance therapy continued for 120 weeks. The 12 years of the event free survival rates for all risk groups j. s 50% ( SD 5% ) .Regarding CNS relapses there was no significant stastitical difference between pts that recei ved 18 or 24Gy. The treatment strategy of GBTLI-82(n=360) from 1982 to 1985, consisted of the same previous induction,consolidation, CNS therapy with cranial irradiation 18 Gy (low risk) or 24Gy (high risk),followed by continuous maintenance for 2 years. The main question in this study was the comparison between sequential rotation or pulses of 3 pairs of drugs during maintenance. At a median follow-up of 10 years, the overall event free survival rates for all children is 58% (SD 4%). There was no significant difference between the two maintenance regimens. The successor GBTLI-85 ran from 1985 to 1988 and registered 431 pts. For the first time no cranial radiation was given to children with very good prognosis. For them, CNS protection was done with triple intrathecal therapy during all treatment. A consolidation therapy with high dose ARA-C was introduced for high risk pts and infants The 6,5 years event free survival for all children is 70% (SD 4%). Significant better results were achieved for high risk and infants pts (EFS 50%). Early intensification therapy and rotational combination chemotherapy improved the outcome in childhood ALL in Brazil.
The Cooperative protocols for childhood ALL treatment started in Brazil in 1980. The first multicenter trial ALL GBTLI-80 directed the main question to CNS treatment. For the group of pts with good prognosis, cranial radiotherapy with 24Gy was compared with 18Gy. At that time, there were no published data in the literature concerning this comparison. In the second study ALL GBTLI-82 fifty three institutions from 13 Brazilian states participated on it. This protocol repeated the classic four weeks induction followed by a consolidation.Pts were randomized during maintenance in two groups:sequential versus pulses therapy. Maintenance treatment duration was 120 wk. According to the third study ALL GBTLI-85 a 6 weeks induction therapy was compared with 4 wk treatment for the intermediate risk group. For high risk pts and infants a HD-ARA-C course (1,5g/m² twice a day during 3 days) was introduced as consolidation therapy. No CNS irradiation was given to children with better prognosis. This third study was closed for pts entry on Dec. 88.
From July 80 to July 82 (ALL GBTLI-80), from Aug.82 to July 85
(ALL GBTLI-82)and from Aug.85 to Dec.88 (ALL GBTLI-85),203, 360
and 431 pediatric pts from 80 different hospitals entered respectively
in these 3 trials. The total number was 994 pts. All children and
adolescents under 18 yr of age with no prior malignancies or no
previous ALL treatment were eligible. Informed consent for the children
to enter those studies were obtained. According to the prognostic
features they were divided in three sub-groups: Good prognosis-
GBTLI-80 included pts with WBC less than 100,000/mm³ , no mediastinal
mass or CNS disease. In next study-82 the criteria was the same,
except for WBC count less than 50,000/mm³ .For the first time, in
GBTLI-85 age and blasts morphology were included as risk factors.
It was included children with 18mo to 10yr of age, L1 morphology
in addition to mentioned-82 criteria.In this group, another sub-set
was considered: pts with less than 10,000 WBC/mm³ at diagnosis (True
Low Risk Group) Poor prognosis- In the study-80 it was included
children of any age,with more than 100,000/mm³ WBC and/or with CNS
disease or mediastinal mass. Next study-82 considered the same criteria
except the WBC above 50,000/mm³ .GBTLI-85 protocol besides the items
defined in the previous study, included age above 10yr and below
18mo, as well as L² morphology.L³ morphology was excluded from these
studies. Intermediate prognosis- In GBTLI-82 and GBTLI-85 were included
in this group pts not eligible in good or poor prognosis. Treatment
Methods- The treatment schedule of the 3 consecutive ALL trials
are outlined in Fig. 1 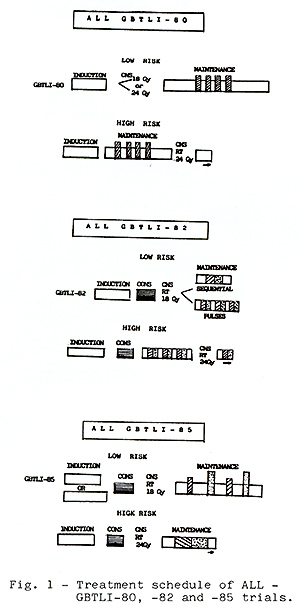
From July 80 to Dec.88 a total of 994 pts from 80 participating
institutions allover Brazil entered in three ALL GBTLI trials. 713
pts were analized. A significant number of institutions were excluded
due to major violations, lack of data and/or bad diagnostic and
supportive care. The results are summarized in Table 1. 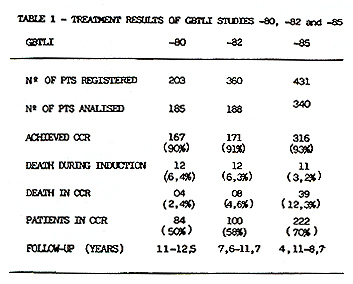 From 713 eligible pts accrued in these studies, 654 (91.7%) achieved CCR. As of July 1992,67 pts have relapsed in study80, 51 in study-82 and 44 in study-85. Most of the recurrences were in the bone marrow. The incidence of isolated and combined CNS relapses was 6,7%, 6,1% and 3,1% respectively in the studies 80, 82 and 85. In this last study 2,9% of the non-irradiated children relapsed in CNS. There was no statistical significant difference between pts that received 18 or 24Gy as cranial radiation in the study GBTLI-80 (p=0,61). The life table analysis for event free survival EFS representing the evaluation of all adverse events (non-responders, deaths and relapses) for all risk groups, according to each study, is depicted in Figure 1. The projected EFS rates are 50% (SD 5%) after 12 years in study GBTLI-80, 58%(SD 4%) after 10 years in study GBTLI-82, and 70%(SD 4%) after 6.5yr of the GBTLI-85. 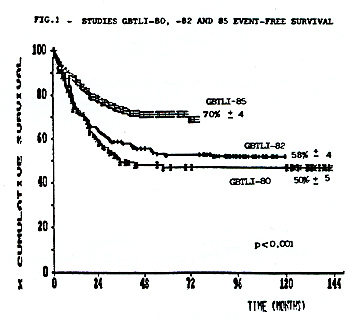 The EFS of protocol-85 according to prognos tic groups reveals that almost 80% of pts with good and intermediate prognosis remained event-free at 6, 5yr, compared with 55% in the group of pts with poor prognosis (Figure 2).There was no statistical significant difference comparing 6wk versus 4wk in the induction treatment for the children with intermediate prognosis. 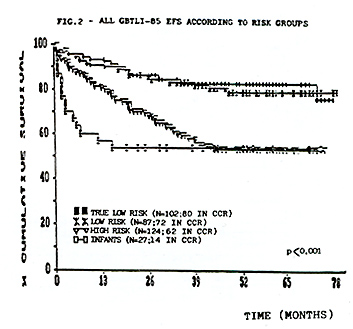 The EFS, according to the initial leucocyte count in GBTLI-85 study, is represented in Fig. 3. 78% of the children with less than 50,000 WBC/mm³while only 50% of those with more than 100,000 WBC/mm³ at diagno sis are in CCR (p=0,0004).According to age 75% of pts between 18mo to 10yr are in CCR at 6,5yr, in comparison with 50% for those below 18mo and above 10yr (Fig.4). In the group beyond 18mo of age (n=27) 11 of them had more than one year at diagnosis. The EFS rates at 6.5yr 62% for those above 12mo being 46% those younger than that. 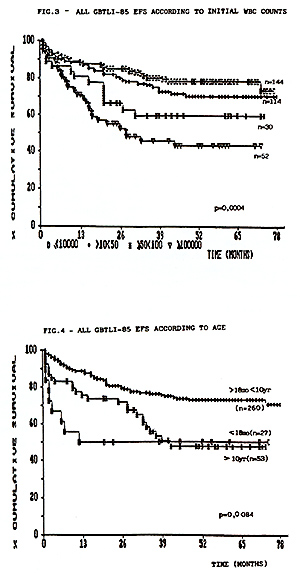
The tremendous regional differences bet ween hospitals' facilities among 80 institutions that registered pts in these 3 studies, were responsible for the restricted number of analized pts. Major violations were the most prominent problem. The CCR in these studies were slightly lower than expected in the literature. This was chiefly due to deaths occuring during induction treatment. In the study GBTLI-85 mortality in remission was unacceptable high (12.3%). This was probably due to the intensive maintenance regimen. Substancial contribution was given to CNS treatment since the study-80. After that we could recommend cranial radiation just with 18Gy for those children with good prognosis. After GBTLI-85, it was possible not to give cranial radiation to pts with very good prognosis. Today the ongoing ALL protocol recommends Cr RT only for pts with more than 50,000 WBC/mm³ at diagnosis. The addition of HD-ARA-C in the consolidation and an intensive maintenance treatment of GBTLI-85 significantly increased the EFS rates for high risk pts compared with previous Brazilian studies -80 and -82 (p=0.0001). The comparison of the latest GBTLI-85 data with other international studies reveals that in Brazil this protocol is able to produce complete remission rates and long term results in childhood ALL, which are comparable to the best results of other studies (1,2,3,4,5).
1. Rivera GK, Raimondi SC, Hancock ML et al.Lancet 1991; 337:61.
|