|
South African Institute for Medical Research and
University of the Witwatersrand. Address for correspondence: SUMMARY Until recently, cALL has been uncommon in sub-Saharan Africa, but there is now emerging a peak of incidence at the age 3 to 5 years in west and southern Africa. Prognosis for African patients with cALL is poor because of a multitude of clinical, biological and social factors. AML is seen at high frequency (probably indicating truly high incidence) in male children 5 .14 years, of whom up to a quarter present with chloroma. It is predicted that the incidence of AML in adults may rise in the near future, related to cigarette smoking, occupational and environmental exposures to benzene and other pollutants, and the prescription of alkylating agents to young people with malignant disease. CML shows no particular epidemiological features, except for a high frequency in young adults and children, reflecting the age structure of the whole population. There are two forms of B.CLL: one is seen most commonly in women of low socioeconomic status towards the end of their reproductive life, and is probably related to an initially polyclonal expansion of B.cells in response of recurrent malaria and other infections; the other is seen over the age of 45 years, with men being affected twice as commonly as women, as in the western world. The study of epidemiology of leukaemias in Africa has been held back by the lack of accurate population census figures, the shortage of haematologIsts and the under-development of laboratories. Therapy is almost always grossly inadequate because of limited supplies of pharmaceuticals, the absence of radIotherapy except in a few centres, and the inability or unwillingness of patients to continue treatment regimes for long periods of time. Despite these handicaps, a large corpus of knowledge has been acquired: this is of global interest, especially in the questions it roses as to epidemIology and possible aetiologies ( 1 ).
Epidemiology There are three epidemiology patterns of incidence of ALL in the world (Table 1) (2). The distinction between epidemiological patterns I and II is certainly wholly false, and is the result of a low rate of diagnosis. Patients with ALL are likely to be dying early of intercurrent infections; they may not attend the hospitals because of a lack of trust, long distances to travel and costs. When patients do attend, the diagnosis may not be entertained in a paediatric clinic where patients commonly present with fever, infection, lymphadenopathy and splenomegaly from other causes. If a peripheral blood film IS examined, the blast cells are likely to be mistaken for activated lymphocytes seen in almost all sick children where malaria is endemic. When the diagnosis is made, there is often no routine for recording and registration of cases. Development of haematology services led to pattern II being reported in Uganda in 1951 (3), in Nigeria 10 years later (4), but not ( disgracefully for the richest sub-Saharan country) in the black population of South Africa until 1975 (5). Where there are experienced haematologists who invest their skills into the diagnosis of ALL in sub-Saharan Africa, the rate of diagnosis rises, but Table 1. Epidemiological patterns of acute
lymphoblastic leukaemia 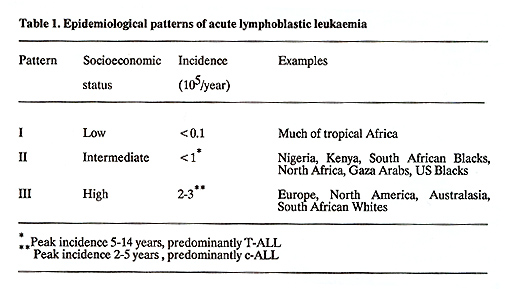
It may be hypothesized that there is a putative childhood leukaemia virus, which is ubiquitous in communities with low levels of hygiene, commonly infects young children causing no or trivial symptoms and is followed by acquired immunity (13): with improving living standards and better hygiene, the mean age of first exposure rises and women experience their primary infections with increasing frequency during pregnancy (14): transplacental transmissIon of the putative childhood leukaemia virus from a non-immune mother to her infant is one step towards the development of cALL (13): within communities with high levels of hygiene, infection during pregnancy and cALL in childhood is more common in first pregnancies, in those of high socioeconomic status (15), in those living in remote areas (16) and following mixing of populations (17, 18). Children of Asian and west Indian ethnic origins living in the United Kingdom have similar patterns of incidence of ALL to white Causasians, an observation which supports the view that environmental factors are more important than genetic (19).
Black African children demonstrate a whole constellation of clinical, biological and sociological features associated with poor prognosis, both in low complete remission rates and short survival (1). Common indicators of poor prognosis include older age, male sex, high initial leucocyte counts, late presentation with central nervous system involvement, mediastinal masses, L2 or L3 blasts, and T -cell phenotype (20). In tropical Africa, all hospital facilities, cytotoxic therapies and radiotherapy are usually substandard or absent (21). Where facilities are adequate compliance is often poor for a variety of reasons, including lack of communication between health professionals and patients, mistrust, long distances to hospital and high costs (22). Multivariant analysis demonstrated that these factors did not totally account for poor prognosis in black Americans, and that there could be some additional undefined host-factors, possible genetically determined (20). In Johannesburg, South Africa, it has been shown that black males with cALL, had the lowest CD10 antigen density, as well as having the worst prognosis: white females had the highest CD10 antigen density and the best prognosis, while the black females and white males occupied intermediate positions: it was hypothesized that the low density CD10 pattern in males and Blacks could be agenetic marker of poor prognosis (23). Regrettably it may be said that patients with ALL in tropical Africa have benefited little or not at all from the great advances which have been made in management during the past decades.
AML is diagnosed at equal frequency as ALL in childhood in sub-Saharan Africa, in contrast to the western world where childhood ALL is seen about four times as often as AML (1,2). The difference in relative rates is due in part to the low incidence of cALL in African children, but also it seems that there is a high incidence of AML; the male to female ratio can approach 4:1, and AML is especially common in boys age 5 to 14 years (Table 2) (24). Between 10% and 25% of children, usually males, have chloromas, most commonly arising in the orbit, in east, west and southern Africa, but this presentation Table 2 Acute leukaemias in children and
adults in northern Nigeria (24). 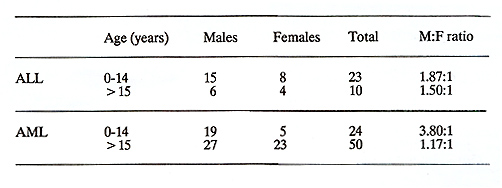
The annual incidence of CML is around 1 per 10 high 5 throughout the world; the male to female ratio is about 1:5:1, with a slightly higher rate amongst black males (29). The peak of frequency of diagnosis is Africa is in the fifth decade, as it is in the western world, but more patients are younger than 40 years than older, reflectIng the age distribution of the population. The disease is not uncommon even in childhood, about 10% of patients in Nigeria and 19% in Sudan being below 15 years of age ( 1, 2). African patients respond as expected to conventional cytotoxic therapy.
Epidemiology Both the gender and age distributions of CLL in tropical Africa differ greatly from those in the western world (30). In west Africa, the male to female ratio is 1:1, as compared to 2:1 in the western world. CLL is common under the age of 40 years and may occur as early as the teens in Africa, whereas it is rare in young western adults. There is arising frequency in women up to the end of their reproductive period of life, and CLL is seen twice as commonly in women than in men below the age of 45 years. Over 45 years, the male to female ratio is : 1, as in the western world. A similar pattern is seen in east Africa, but is not so pronounced. All the younger patients with CLL are of low socioeconomic status or come from rural areas.
It is postulated that the unique pattern of CLL in tropical Africa is the result of recurrent malaria and other infections, leading to both a depression of T -cell control of B-cell proliferation and direct antigenic and mitogenic stimuli to B-cell proliferation: there is a greatly enlarged polyclonal B-cell pool, especially in subjects living in rural areas and in poor hygienic conditions: the physiological depression of cell mediated immunity of normal pregnancy leads to an increased susceptibility to malaria, and also to certain bacterial and viral infections, as well as reducing surveillance for somatic mutations: all these factors ( endemic malaria, the transmission of other infections which could include oncogenic viruses, numerous pregnancies) favour the proliferation of a large pool of B-cells in which there is a relatively high chance of somatic mutations leading to a monoclonal proliferation of B-CLL. The hyper-reactive malarial splenomegaly syndrome (HMS), previously called the tropical splenomegaly syndrome (TSS), is a condition in which there is probably a genetically determined malarial-triggered suppression of T -cell control of B-cell proliferation, resulting in a grossly exaggerated B-cell response to recurrent malaria: the chances of developing CLL should be in theory greater in patients with HMS than in the general population and it has been demonstrated recently that clonal lymphoproliferation can complicate HMS, and could lead to the evolution of CLL or splenic lymphoma with villous lymphocytes (31,32).
1. Fleming AF. Leuk Res 1986; 10: 1353. |