|
|
|
Introduction In recent years hematopoietic growth factors have been investigated with dose-intensive chemotherapy protocols without stem cell rescue. Neidhart et al. [9, 10] treated patients with G-CSF or GM-CSF after repetitive courses of high-dose chemotherapy consisting of three non-cross-reacting cytotoxic drugs (cyclophosphamide, etoposide, and cisplatin); the period of severe neutropenia was significantly reduced by growth factors as compared to patients who were treated without cytokines. Several other investigators also reported a reduction of neutropenia by growth factors following doseintensified regimens [4,8,11]. All these studies demonstrated that high-dose chemotherapy is feasible without bone marrow transplantation (BMT) , althourgh there is still a 2- to 3-week period of severe cytopenia, particularly severe thrombocytopenia. To further reduce the period of cytopenia, the use of peripheral blood progenitor cells (PBPC) was investigated. Those cells have been effectively mobilized following high-dose cyclophosphamide and GM-CSF [3]. PBPC were used for autografting and were shown to induce a more rapid recovery of neutrophils and platelets when compared to BMT, probably due to high numbers of committed progenitor cells retransfused (for Review see [7] ) . In this study we investigated the requirements for combining a standard dose chemotherapy regimen (YIP) with the mobilization of PBPC with different growth factors or growth factor combinations (GM-CSF versus IL-3 + GM -CSF) .The idea was to harvest PBPC following a I-day course of YIP chemotherapy (YP 16 500 mg/m², ifosfamide 4000 mg/m², cisplatin 50 mg/m²) plus growth factor adminstration and to retransfuse those cells into patients after they have received a high-dose, 3-day YIP chemotherapy regimen (cumulative doses of YP16 1500 mg/m², ifosfamide 12000 mg/m², cisplatin 150 mg/m²) .The phenotypical and functional characterization of PBPC mobilized under different conditions are described here .
Thirty-two cancer patients with refractory tumors or patients with
samll cell lung cancer were treated with a I-day course of YIP chemotherapy.
Growth factor application was started 24 h after the cnd of chemotherapy,
either with s.c. GM-CSFalone (days 1-15) orwith a combination ofIL-3
(days 1-5) and GM-CSF (days 6-15). The numbers of peripheral blood
clonogenic progenitor cells [myeloid (CFU-GM), erythroid (BFU-E),
and multi potential (CFU-GEMM)] were analyzed during the reconstitution
phase following YIP chemotherapy using in vitro culture techniques
in semisolid media [5]. The number of circulating CD34 + cells was
determined by immunoperoxidase staining methods [1]. Dual color
flow cytometry analyses ofCD34+ cells were performed for the further
characterization of recruited PBPC. Using the combined IL-3 + GM-CSF
application following YIP chemotherapy, median levels of more than
10 000 CFU-GM/ml blood (range 1000-24300) were recruited, corresponding
to a median of 418 CD34+ cells/µl blood (range 106-1841; [2]). In
parallel, large numbers of erythroid (median of 10 600 BFU-E/ml
blood) and multipotential (median of 640 CFU-GEMM/ml blood) progenitor
cells were recruited in IL-3 + GM-CSF treated patients. In GM-CSF
treated patients, however, significantly fewer progenitor cells
of all lineages were recruited. Total clonogenic progenitor cells
mobilized with different growth factors are illustrated in Figure
1. Dual color flow cytometry analysis of CD34 + cells revealed that
the majority of CD34+ cells coexpress CD33, CD38, or HLA-DR molecules,
indicating that these cells are committed progenitor cells rather
than very primitive hematopoietic progenitor cells. Approximately
10 %-30% of CD34+ cells lack the expression of CD33, CD38, or HLA-DR
antigens; these cells might comprise pluripotent stem cells. One
representative flow cytometry analysis of a patient treated with
IL-3 + GM-CSF is shown in Figure 2. 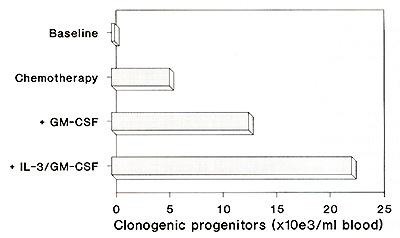 Fig. I. Total numbers of clonogcnic progcnitorcells per milliliter blood following l-day YIP chemotherapy with or without growth factors. Data arc presented as median maximum values of a total of 46 chemotherapy cycles 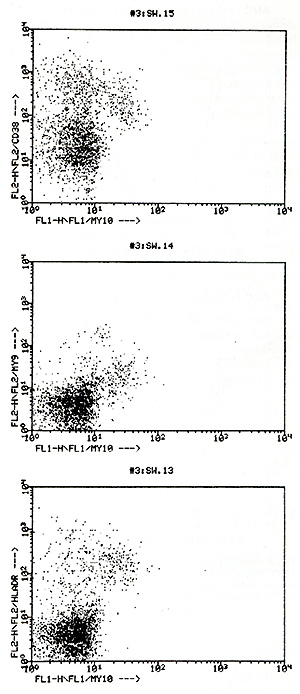 Fig. 2. Dual color flow cytometry analysis of patient treated with IL-3 + GM-CSF following YIP chemotherapy. Data are presented in a dot-plot analysis of My lO (CD34) fluorescence versus My9 (CD33), CD38, or HLADR fluorescence 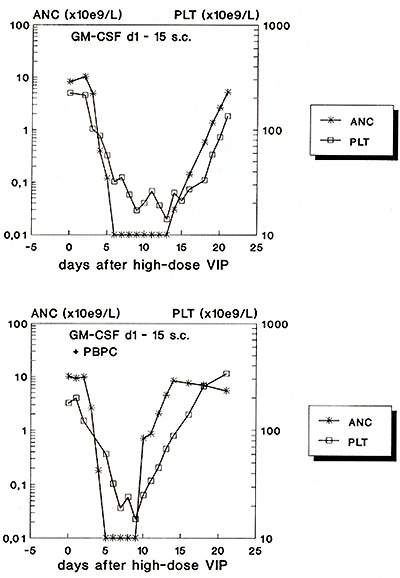
Regression analyses of CD34 + cells versus clonogenic progenitor cells revealed a good correlation with myeloid progenitor cells in GM-CSF treated patients. In IL-3 + GM-CSF treated patients, however, no strong correlation between CD34 + cells anc clonogenic progenitor cells was observed. The lack of linear correlations demonstrate that the rise in CD34 + cells is not necessarily accompanied with an increase in clonogenic precursor cells. These observations again suggest that part of CD34 + cells represent relatively mature cells without clonogenic capacity in vitro [ 6] . Our data show that YIP chemotherapy plus IL-3/GM-CSF treatment allowed to recruit circulating CFU-GM in numbers comparable to data published with high-dose cyclophosphamide + GM-CSF [3]. Using the mobilization technique described, we have recently started to collect sufficient numbers of PBPC by one single leukapheresis in pilot patients and retransfused them following high-dose 3-day YIP chemotherapy. Hematopoietic recovery of two representative patients treated with or without PBPC are show in Figure 3. Recovery data on ten patients treated with or without PBPC support revealed a significant reduction in the duration of neutropenia as well as thrombocytopenia in patients treated with peripheral blood progenitor cells as compared to patients who were treated with growth factors alone . In conclusion, standard dose YIP chemotherapy plus hematopoietic growth factor treatment recruits large numbers of predominantly committed PBPC which might be effective in the reduction of severe pancytopenia following high-dose YIP chemotherapy. Phase II studies in patients with chemosensitive tumors or lymphomas are ongoing at our institution to demonstrate the clinical efficacy of such an approach.
1. Bross KJ, Pangalis GA, Staatz CB, Blume KG (1978) Demonstration
of cell surface antigens and their antibodies by the peroxidase-anti-peroxidase
method. Transplanta tion 25:331 |