|
1 University of Texas M.D., Anderson Cancer Center, Department of Pediatrics, 1515 Holcombe Boulevard, Houston, TX 77030, USA. Since the first case report in 1959 of total body irradiation and bone marrow transplantation (BMT) in a child with acute leukemia, the popularity of this approach has steadily increased [1]. The introduction of human leukocyte antigen typing and mixed leukocyte cultures and improved methods of supportive care made BMT a successful way of treating certain immunodeficiency disorders, severe aplastic anemia, chronic myeloid leukemia, and certain other blood dyscrasias as well as acute leukemia [2]. The purpose of this essay is to examine critically the practice of myeloablation and marrow transplantation in children with acute leukemia.
The first generally accepted use of allogeneic BMT in children
with leukemia was for those with acute myeloid leukemia (AML) in
hematological remission after initial chemotherapy [2]. It was thought
that relapse was almost inevitable in these patients, so the reports
of apparently permanent remission after BMT convinced most hematologists
that BMT was the treatment of choice if a histocompatible sibling
donor were available. However, in the past 10 years it has become
apparent that combination chemotherapy alone without BMT may be
as effective as BMT. This is reflected in a 1989 statement of the
International Bone Marrow Transplant Registry (IBMTR): "It is not
known whether chemotherapy or bone marrow transplantation is the
more effective treatment for acute myelogenous leukemia in first
remission " [3] . A recent 6-year follow-up report from St. Jude
Children's Research Hospital of a 1980-1984 study of therapy of
AML describes no significant difference in 6year continuous complete
remission rates between BMT and chemotherapy [4]. Nine of 19 BMT
patients remained in continuous complete remission for a median
of 68 months and 13 of 42 chemotherapy patients for a median of74
months (Fig. 1 a). Similar experience has been reported from the
Johns Hopkins Oncology Center in young adults with AML [5]. When
corrected for patient selection, their data indicate that the frequency
of lengthy continuous complete remissions was similar for 41 patients
treated with allogeneic BMT and 46 patients who received intensive
chemotherapy without BMT (Fig. 1 b ). From January 1986 to February
1989 the Children's Cancer Study Group admitted 617 children with
AML into a study in which those with histocompatible sibling donors
underwent BMT after remission induction while the others received
intensive chemotherapy for 3 months with or without subsequent maintenance
chemotherapy [6]. The actuaria12-year event-free survival from the
time of BMT or intensive chemotherapy is not significantly different,
41% for intensive chemotherapy and 50% for BMT (p = 0.41). It is
possible that late deaths from chronic graft vs. host disease and
its complications, secondary neoplasms, or late relapses might modify
this outcome in favor of one or the other methods. 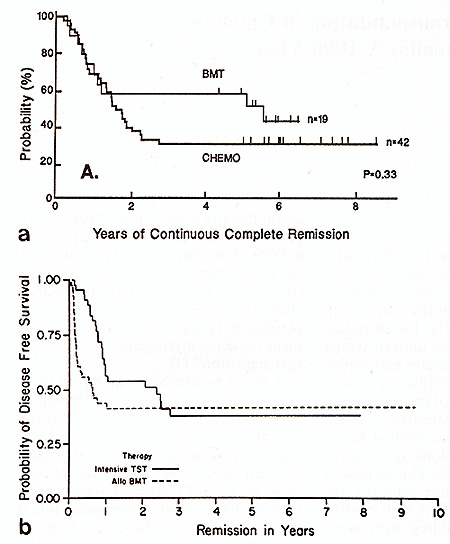
Acute Lymphoid Leukemia at High Risk of Relapse
One of the early publications concerning allogeneic BMT is second remission of ALL concluded that "marrow transplantation offers the best chance of long term remission and potential cure after a child with ALL has had a relapse in the marrow" [10]. This was based on a nonrandom comparison in which 9 of 24 children survived in remission after BMT and only 1 of 21 after chemotherapy alone. However, scrutiny of the published data reveals that 11 of the 24 BMT patients had isolated extramedullary relapse, which has a more favorable response to treatment [11], rather than marrow relapse. This contrasted with 4 of 21 chemotherapy patients who had extramedullary relapse. The median duration of first remission, an important prognostic factor for second remission [12], was 25 months for BMT patients and 13 months for chemotherapy patients. Finally, the delay between remission induction and BMT ranged up to 17 months, thus tending to exclude patients with early relapse and, therefore, the worst prognosis. In retrospect, the data did not justify the conclusion. A recent report comparing allogeneic BMT vs. chemotherapy without BMT in children with ALL in second remission attempts to address the problems of other such comparisons [13]. The patients who received chemotherapy alone had "risk factors" for relapse comparable to the BMT patients and had been in complete remission for 2-3 months prior to entry into the study. However, BMT was delayed up to 13 months and no description is given of the drug schedules and medical care of the chemotherapy patients. For these reasons the reader cannot be certain whether the superior outcome of BMT was related to the exclusion of patients with early relapse, the most reliable prognostic factor. Also, one is unable to assess whether medical care was comparable in the two groups and whether the chemotherapy alone patients received optimal drug therapy.
There is a surge of interest in treating childhood acute leukemia
with myeloablation and auto grafting of cryopreserved bone marrow
obtained during hematological remission and subjected to "purging"
with biological or chemical agents. A recent report concludes that
this may be a treatment option for children with ALL in second or
subsequent remission whose first remissions were longer than 24
months [14]. Of44 patients grafted, 15 were in continuous second
remission 1094 months; all 15 had initial remissions longer than
24 months. Children with T cell ALL or B-precursor ALL without CD
10 or CD 9 surface antigens were excluded. Delays of 1-11 months
between remission and autograft excluded other patients with more
aggressive or resistant leukemia. The event-free survival was similar
to that reported previously for a group of28 children with ALL in
second hematological remission treated with chemotherapy alone [12].
In both the autograft and the chemotherapy alone series those with
brief initial remissions had short second remissions while those
with long first remissions had longer and sometimes durable second
remissions (Fig. 2). There is no evidence that adding a marrow autograft
procedure to chemotherapy changed the outlook for survival. In contrast
to the chemotherapy only regimen, all longterm survivors of the
autograft procedure had growth failure and 9 of 28 patients in remission
3 months after grafting experienced hemolytic-uremic syndrome. A
report from the IBMTR summarizes their view: "Whether auto transplants
are equivalent or superior to other therapies. ..is uncertain, since
prospective trials are not reported and data analysis is confounded
by selection of subjects and time-censoring" [ 15] . 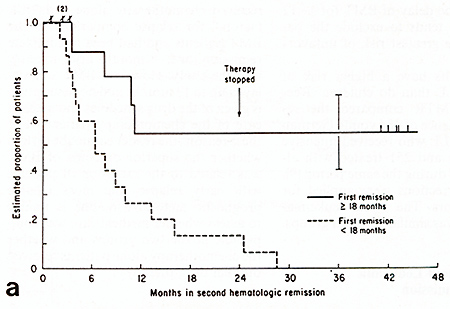 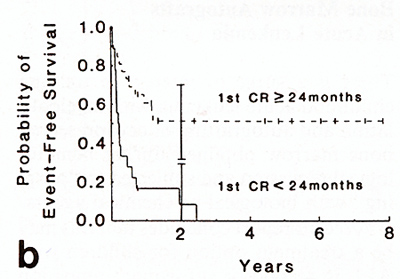
From the earliest reports of curative approaches to children with acute leukemia pediatricians have been concerned about the quality of survival. Studies have focused on anthropometric and neuropsychological measurements of surviving children. Cure has been defined as not only eradication of leukemia but restoration of normal health and normal capacity for physical, intellectual, social, and emotional growth and development. The need for weighing the value of each component of treatment against its ultimate risk to normal health, growth, and development of the children has been emphasized. Children who survive leukemia have the potential for a much longer life than adult survivors and thus a longer period of risk for delayed effects such as second cancers or organ failures. In addition, the growing tissues and more rapidly replicating cell systems of children are more vulnerable to cytotoxic agents. For example, preschool children are more likely to experience neuropsychologic deficits after cranial irradiation than older children and adults [16]. Children's hearts are apparently more vulnerable to delayed anthracycline cardiomyopathy than adults [17]. For these reasons one must necessarily be concerned about the late effects of treatment of children. There are relatively few descriptions of the delayed sequelae of BMT. Growth failure is universal in the Seattle series, probably as a result of total body irradiation [18]. Survival must therefore be considered dysfunctional despite the courage and vigor of the children, their families, and their physicians in overcoming the problem. This contrasts with the outcome of chemotherapy alone in which "catch-up" growth usually occurs after cessation of therapy in those children whose growth is slowed on treatment [19]. Although gonadal failure may follow treatment with alkylating agents, the majority of children with leukemia receive little or no drugs of this class and fertility is usually preserved [20]. In contrast, approximately 70% of BMT survivors experience gonadal failure [18,21]. Other endocrine deficiencies, rare in chemotherapy survivors, are reported in about one third of BMT survivors. Chronic graft vs. host disease occurs in approximately one third of children after allogeneic BMT [18, 21]. This can result in crippling organ failure as well as a continuous risk of life-threatening infection. Obstructive and restrictive pulmo nary disease, often fatal, is another complication of BMT not seen in children with leukemia treated with chemotherapy alone [22] . Second malignant solid tumors 10-30 years later are among the delayed sequelae of childhood cancer. Some may be related to the first neoplasm but the greatest risk appears to arise from treatment with radiation therapy and alkylating agents [23]. The administration of total body irradiation and high dosages of alkylating agents such as busulfan and cyclophosphamide are customary methods of myeloablation in marrow transplant and autograft procedures. Given the long life expectancy of children cured of cancer and the carcinogenic effects of radiation and alkylating agents, it can be anticipated that children with leukemia treated with BMT or auto grafts will experience a very high incidence of malignant solid tumors as young adults. In summary, available data indicate that the human "price of cure" is appreciably higher in children treated with BMT than with current chemotherapy regimens.
The difficulties in comparing outcomes of alternative treatments of cancer are well known. Among them are patient selection, lack of randomization, enthusiasm for test therapy, differences in quality or level of medical care, misuse of survival curves, and failure to describe fully the sequelae of treatment so that its human cost can be compared to its benefits. In the evaluation of reports of BMT in acute leukemia of children there are some specific problems [24]. First is the exclusion of potentially eligible patients. BMT is usually performed during hematological remission. Therefore, patients who fail to experience remission are excluded from the procedure. Because of delays between remission and the BMT procedure patients who experience relapse prior to BMT are also excluded. Since failure to enter remission and early relapse tend to signify more resistant, more aggressive leukemia with poor prognosis for survival, these exclusions are highly selective for providing BMT candidates that have a relatively favorable outlook. An example of this selective process was demonstrated in the Pediatric oncology Group (POG) 8710 study of treatment of children with ALL in first hematological relapse [25]. Of lOO patients registered in the study, 74 had HLAtyping. Of 16 children found to have a fully matched sibling donor, only seven underwent BMT. The other nine children either failed to experience a second hematological remission or suffered another relapse before BMT could be performed. Thus, one half of the eligible patients, the half with the worst outlook for survival, were excluded from BMT . The effect on apparent therapeutic outcome of excluding patients who have early relapses from BMT can be appreciated by consideration of expected failure rates for patients under 21 years of age during the first few months after remission induction of acute nonlymphoid leukemia (ANLL) [26]. Almost one fifth of patients experience relapse during the first 3 months of remission. Therefore, any intervention introduced after 3 months of remission will be followed by an apparently better relapse-free survival than no intervention because these early relapses are discounted. If a comparison is made between patients who receive the intervention and cohorts who do not, the relapse-free survival of those who do not receive the intervention will appear to be less because their number will include all the patients who experienced relapse in the first 3 months. In other words, the apparent result of a delayed intervention looks favorable for two reasons -exclusion of early relapse patients from the intervention group and their inclusion in the nonintervention group. An example is the initial comparison ofBMT vs. chemotherapy for continuing remission of ALL in second hematological remission in the POG study 8303 [27]. A marginal superiority was noted for BMT with regard to remission duration. However, remission duration was measured from remission induction for the chemotherapy patients and from time of BMT, 3-28 weeks later, for BMT patients. Thus, early relapse patients reduced the apparent failure rate of BMT and increased the apparent failure rate of chemotherapy without BMT.
In determining the value of alternative therapeutic interventions in childhood acute leukemia, two questions need to be answered. Which treatment results in the higher cure rate, and what is the relative cost/benefit ratio of the treatments? At present there is no demonstration of superior survival of children treated with allogeneic BMT for ANLL in first remission, ALL with "unfavorable prognostic factors" in first remission, or ALL in second remission. There is also no demonstration of superior survival with bone marrow autografts. At the same time, the immediate toxicity and late sequelae of these procedures are clearly greater than with modern chemotherapy, especially current successful protocols that avoid or minimize use of radiation therapy, anthracyclines, and alkylating agents [28]. For these reasons BMT and autograft procedures in children with acute leukemia need to be reserved for experimental investigations in those leukemias and preleukemias that are clearly demonstrated to be usually fatal with current chemotherapy regimes. Secondly, the investigations should be collaborative and prospective with randomization for BMT immediately prior to myeloablation, optimal graft procedures and chemotherapy regimes, and comparable specialized medical care. Just as important, there must be complete accounting and description of the health and growth of survivors as well as meticulous data analysis and reporting.
|