|
The Fred Hutchinson Cancer Research Center and
the University of Washington School of Medicine 1124 Columbia St.,
Seattle, WA, 98104, USA
The use of allogeneic marrow transplantation as treatment for patients with various hematological diseases has increased in recent years [1-6]. A survey by the International Bone Marrow Transplantation Registry [7] estimated the number of transplants carried out through the year 1987 to be in the order of 20000, more than 10000 of these during the years of 1985 through 1987. Marrow transplantation has been employed in most cases ( > 80%) for therapy ofmalignant hematologic diseases. Roughly 10% of all transplants have been for the treatment of patients with acquired or inherited marrow dysfunction (aplastic anemia), and 5% -6% have been for treatment of congenital defects of the hematopoietic and immune systems (thalassemia major, severe combined immuno-deficiency disease, and other inborn errors). Since 1970, when marrow transplantation was restricted to patients with advanced hematologic malignancies and disease-free survival was in the order of 15%, remarkable advances have been made [8]. Recent studies in patients with acute nonlymphoblastic leukemia (ANL) in first chemotherapy-induced remission have shown actuarial survival to be superior in patients undergoing marrow transplantation (50%) compared with those given chemotherapy (20% ) with a follow-up period of up to 10 years. Patients with acute lymphoblastic leukemia (ALL) given grafts in second or subsequent remission have shown disease-free survival of approximately 35%, whereas patients undergoing chemotherapy all died of recurrent disease within 3 1/2 years of the initiation of therapy. Fifty to 60% of patients with chronic myelocytic leukemia (CML) transplanted while in chronic phase have obtained disease-free survival whereas none can be cured with chemotherapy alone. In patients with aplastic anemia treated with marrow grafting, survival has improved to 60% 80% compared with 40% 50% for patients treated with immunosuppression by anti thymocyte globulin and only 20% for patients who receive only supportive therapy. Marrow grafting has produced 70% disease-free survival in patients with thalassemia major [9] and approximately 50%-60% survival in patients grafted for severe combined immunodeficiency disease and other inborn errors [10]. Despite impressive improvements, major problems and complications in marrow transplantation remain [16, 8, 10 23]. These are listed in Table 1. In patients grafted for leukemia 17% -75% of treatment failures are attributable to relapse, whereas graft rejection has resulted in the death of 5% -12% of patients grafted for aplastic anemia. Significant acute graft-versus-host disease (GVHD) with a case fatality rate of approximately 50% is seen in 18% -45% of all patients and it is responsible for 10% -25% of deaths. Fatal interstitial pneumonias are often associated with acute GVHD or may be the result of drug and radiation toxicity. Methods of improving the results of marrow transplantation are needed.
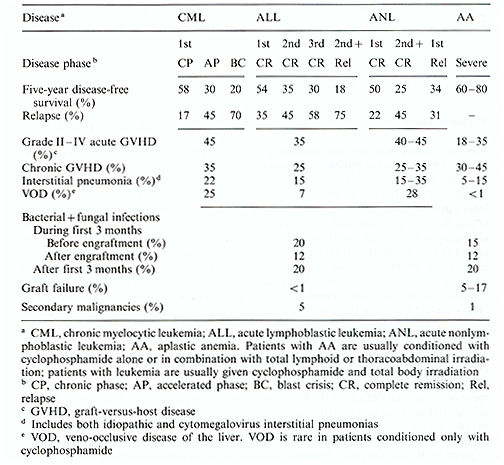
Table 2. Effect of T -cell depletion on
incidence of GVHD and graft failure in patients transplanted for
leukemia. 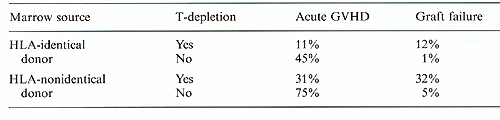 Table 3. Leukemic relapse and T-cell depletion. (Data from the International Bone Marrow Transplant Registry[28]) 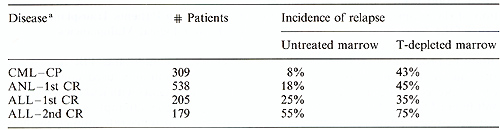
A common problem in patients given HLA-identical marrow grafts for the treatment of severe aplastic anemia after conditioning with high-dose cyclophosphamide has been graft failure [3, 5, 21, 30]. In the early 1970s this problem was seen in 30% -60% of patients. Two factors were associated with rejection: positive in vitro tests of cell-mediated immunity, indicating reaction of host lymphocytes against antigens on donor cells before transplantation; and, secondly, a low number of transplanted marrow cells ( < 3 x 10 high 8 cell/kg). As supported by studies in experimental animals, immunity of recipient against donor is thought to be the result of transfusion-induced sensitization. Canine studies have indicated that dendritic mononuclear cells in transfused blood products lead to sensitization of the recipient against minor antigens of the donor which may not be suppressed by the immunosuppressive conditioning programs [31]. Transplants carried out in patients who have not received preceding transfusions rarely result in graft failure: 80% of untransfused patients are alive with functioning grafts. This suggests that immunological mechanisms involved in graft failure are, for the most part, induced by previous blood transfusions. Many regimens, mainly involving more intensive immunosuppression, are being used to avoid graft rejection in multiply-transfused patients. All programs include cyclophosphamide, but other features vary, such as the use of total body irradiation (TBI), total lymphoid irradiation, total nodal irradiation, and thoracoabdominal irradiation. The Seattle team has administered viable donor buffy coat cells along with the marrow infusion, since the donor's peripheral blood is a potential source of hemopoietic stem cells and/or lymphoid cells capable of abrogating rejection. Most transplant centers are now reporting that rejection rates have decreased and survival has increased in multiplytransfused patients with survivals between 60% and 70%. Risks are associated with most of the conditioning programs. Huffy coat cells may lead to an increase in chronic GYRD. Irradiation carries the potential for future cancer. Because of these risks as well as the persistent possibility of rejection, emphasis should be placed on preventing rather than overcoming sensitization caused by blood transfusions. This is best done by performing transplantation before administering transfusions. In case transfusions are required, buffy coat-poor red blood cells and platelets should be used. Recent data in the canine model have shown that sensitization can be prevented if blood transfusion products are exposed to ultraviolet light irradiation [32].
Cyclophosphamide and THI have been the most commonly used conditioning agents for patients with leukemia [1-4,8, 1113]. In the attempt to reduce the leukemic recurrence rate, numerous therapeutic reagents such as etoposide, highdose cytosine arabinoside, piperazinedione, BCNU, and others have been used in addition to or instead of cyclophosphamide. Fractionated TBI has slowly replaced single-dose TBI over the past decade since a prospective comparison of the two schedules showed fractionated TBI to be better tolerated and to result in fewer long-term complications without any apparent increase in postgrafting relapse rates [33]. Hyperfractionated TBI followed by cyclophosphamide has been used in patients with ALL in second or subsequent remission by the Sloan-Kettering team with apparently superior results [11]. A combination of busulfan and cyclophosphamide has been used without TBI by the Johns-Hopkins team and they reported very low leukemic recurrence rates in patients with ANL in first remission, while relapse rates in patients with more advanced ANL appeared to be similar to those seen after cyclophosphamide/TBI regimens [14]. This appears to contrast with results reported by the Ohio State team, which suggest that relapse rates are low not only in patients with ANL in first remission but also in patients with advanced ANL and ALL, even with reduced doses of busulfan and cyclophosphamide [34]. It appears, however, that the limits of nonhemopoietic toxicity have been reached and no substantial improvements in relapse rates and survival can be expected using systemic chemotherapy and TBI. In principle, the most efficient means of eradicating cancer would be to use agents which interact specifically with malignant cells. The method approaching this ideal most closely is the use of monoclonal antibodies directed against tumor-associated antigens. It is known that monoclonal antibodies injected in vivo can concentrate on tumor cells; however, the antitumor effect is limited, partly due to the fact that some tumor cells lack target antigens, and partly because some cells, though coated by antibody, may not be killed by it. Attempts are being made to link antibodies to toxins such as the ricin-A chain for more effective tumor cell kill. Also in progress are studies attaching monoclonal antibodies to short-lived radioactive isotopes which deposit most of their energy within a 1- to 2-mm radius. With these isotopes, cells expressing the target antigens as well as neighboring cells which may be antigen negative will be killed. In the case of hematologic malignancies, subsequent marrow "rescue" would be needed since this approach would ablate normal marrow cells. Initial experiments in a canine model of marrow transplantation have shown appropriate antibody isotope conjugates to localize preferentially in the marrow and spleen and also, to a lesser extent, in lymph nodes [35, 36], with the amount of isotope in the marrow achieving a ratio of 5: lor better as compared with other organs. The marrow aplasia caused by radiolabeled antibodies can be reversed by infusion of cryopreserved autologous marrow at a time when very little radioactivity is left, about 8 days later. Canine studies are underway exploring the efficacy of various combinations of chemotherapy, TBI, and radiolabeled antibodies in conditioning dogs for T -cell-depleted marrow grafts. It is anticipated that refinements of this approach, particularly the use of high-energy beta-emitting isotopes with short linear energy transfer, will lead to less toxic and more efficient conditioning programs which will not only provide better elimination of malignant cells but will also ameliorate the problem of graft failure. Radiolabeled antibodies might be useful in transplantation for nonmalignant as well as for malignant hematological diseases, by allowing engraftment to take place while eliminating busulfan in patients with thalassemia major or reducing the dose of cyclophosphamide in patients with aplastic anemia.
Interstitial pneumonias are among the most serious complications arising during the first 3- 4 months after transplantation (reviewed in [23, 37,38]). Pneumonias are less frequent in patients grafted for aplastic anemia following cyclophosphamide than in patients with leukemia whose conditioning regimen included TBI or busulfan. Pneumocystis carinii infection, formerly the cause of about 10% of all interstitial pneumonias, is now being prevented by prophylactic trimethoprim sulfamethoxazole. Idiopathic interstitial pneumonia has been seen in approximately 13% of patients given single-dose TBI, but the incidence has declined to 3% with the use of fractionated TBI. By far the most critical infection is cytomegalovirus (CMV). Evidence of CMV activation is seen in about 75% of all patients with positive CMV antibody titers before transplant. While often asymptomatic and manifested only by viral excretion in the urine or by increasing antibody titers, CMV activation can develop into a serious complication in the form of CMV pneumonia, which has a case fatality rate of approximately 85%. Patients who are CMV seronegative before transplant can be protected from infection by the use of CMV -sero-negative blood products during and after transplant. If possible, only CMV -negative blood products should be given to any CMV -negative patient who is a potential transplant candidate. Immunoprophylaxis using CMV immunoglobulin has been controversial, and there is currently no proven therapy for established CMV infection. The use of an acyclovir derivative, dihydroxymethyl-ethoxymethylguanine, has not been effective in treating CMV pneumonia although it has significantly reduced the amount of virus in the lung tissues, and it may prove to be beneficial when given along with CMV immunoglobulin in treating established CMV pneumonia. Also, it may be useful in prophylactic trials. It is possible that the use of certain recombinant human hematopoietic growth factors, such as IL-l, IL-3, GCSF, and GM-CSF, might shorten the period of granulocytopenia or thrombocytopenia after grafting, thus reducing the incidence of early infection and resulting in a modest improvement of survival.
In the early 1970s marrow transplants were only administered to patients who had advanced acute leukemia, severe aplastic anemia, or severe combined immunodeficiency diseases. Since then, the technique has been shown to be beneficial and even curative for patients with many different hematological conditions. In younger patients, marrow grafting is now the treatment of choice for aplastic anemia, immunodeficiency disease, certain genetic disorders of hemopoiesis, any leukemia which has relapsed at least once, ANL in first remission, and CML. For patients who have thalassemia major, CML in chronic phase, or ANL in first remission, the risk of early death from transplant-related complications must be weighed against the benefit of long-term cure. Although impressive advances in transplantation have taken place, major problems persist. These include recurrence of leukemia, graft failure in patients given T -depleted or HLA-nonidentical grafts, acute and chronic GVHD, infections associated with prolonged immunodeficiency, and late-occurring complications resulting from the conditioning programs. Major improvements in the area of more effective and less toxic conditioning regimens are needed. In this regard, the use of monoclonal antibodies linked to short-lived radioactive isotopes with short linear energy transfer seems promising. It is expected that more effective conditioning programs will decrease the incidences of leukemic recurrence and graft failure. Better conditioning regimens should permit a broader application of T -cell depletion to prevent acute and chronic GVHD, thus extending marrow grafting to include more HLA-non identical and unrelated patients. The use of recombinant hemopoietic growth factors may prove to reduce the risk of early infections, but the problem of CMV infection in seropositive recipients will remain until effective antiviral drugs are identified.
1. Gratwohl A, Hermans J, Barrett AJ, Ernst P, Frassoni F, Gahrton
G, Granena A, Kolb HJ, Marmont A, Prentice HG, Speek B, Vernant
JP, Zwaan FJ (1988) Allogeneic bone marrow transplantation for leukaemia
in Europe: report from the working party on leukaemia, European
Group for Bone Marrow Transplantation. Lancet I: 1379-1382 |