|
1 These studies were supported under Grants CA-23175.
CA-12800. and CA-15688 from the National Cancer Institute. and Grant
RR-00865 from the U.S. Public Health Service.
Acute leukemia is a neoplastic disease characterized by the abnormal proliferation and accum illation of immature hematopoietic cells. Progress in our understanding of this disease is reviewed in this volume. Significant recent advances in the therapy of acute leukemia include high remission rates in both acute lymphoblastic (ALL) and acute myelogenous leukemia (AML), the prevention of central nervous system leukemia in ALL, and development of moderately effective remission maintenance programs, particularly in ALL. Despite this progress, approximately 50 per cent of patients with ALL and over 95 per cent of those with AML will eventually die of resistant leukemia. Recent studies at our institution and others have clearly demonstrated the feasibility of transplantation of normal hematopoietic stem cells in man. In view of this, and because of the disappointing results of chemotherapy in patients with acute leukemia who relapse, we studied the potential role of bone marrow transplantation in resistant acute leukemia. In this chapter I will briefly review some basic aspects of the biology and immunology of marrow transplantation and discuss its applicability to leukemia.
The hematopoietic system is derived from pluripotent stem cells. These cells have several inherent characteristics relevant to marrow transplantation including self-renewal potential, differentiative capacity, and the presence of histocompatibility antigens (HLA) on their surface. It is clearly possible to transplant hematopoietic stem cells in man. Requirements for engraftment include histocompatibility matching between donor and recipient, immunosuppression to prevent graft rejection, and a critical dose of marrow cells. The latter may relate to the clinical setting under which transplantation is performed rather than an inherent characteristic of stem cell(s) since a single cell may be capable of repopulating a congenitally anemic non-irradiated mouse under appropriate conditions. The human major histocompatibility complex (MHC), referred to as HLA, has been assigned to chromosome 6. The HLA locus has been further subdivided into the HLA-A, B, C, and D subloci. The first three are commonly defined by serologic technics while the HLA-D region is conventionally studied in the mixed lymphocyte culture (MLC) test (for review see [ 1 ]). Successful marrow transplants in man have been restricted to HLAidentical siblings with few exceptions. While HLA is of prime importance in determining graft outcome, other histocompatibility systems are undoubtedly involved. Little is known regarding these non-HLA systems and no attempt has been made to match for non-HLA antigens in clinical transplantation. This factor probably accounts for the high incidence of graft rejection and graft-versus-host disease (GVHD). There is a high degree of polymorphism in the HLA system. Since these antigens are inherited in a Mendelian fashion as codominant alleles, there is a reasonable possibility (25 per cent) of finding an HLA-identical donor within a family. In the general population the probability is in the range of one in 10.000. Because of this, most transplants have been performed between HLA-identical siblings. Despite profound hematopoietic suppression, patients with aplasia and acute leukemia are capable of rejecting allogeneic grafts. Immunosuppression is therefore necessary to achieve sustained marrow engraftment. Pretransplant immunosuppression, referred to as conditioning. has utilized chemotherapy and radiation either singly or in combination. Since doses used in these regimens are supralethal, rescue with normal marrow is essen tial for survival. The transplant procedure is relatively simple. Approximately one liter of bone marrow is removed from the donor by aspiration from the posterior iliac crests. A single cell suspension is prepared and infused intravenously to the recipient. The infused cells home to the marrow after a brief delay in the lungs and spleen. The usual dose is 1-5 X 10 high 8 nucleated marrow cells per kg. In most instances discrete clusters (colonies) of hematopoiesis are observed in the marrow with the first 2 weeks following transplantation [3]. These clusters are usually either erythroid or granulocytic, but mixed populations are occasionally observed. Peripheral white blood cells and platelets begin to rise within 2-3 weeks following transplantation and may return to normal levels by 1-2 months. Cytogenetic and gene marker studies clearly indicate that red cells, granulocytes, lymphocytes, platelets, monocytes, and hepatic and alveolar macrophages are ofdonor origin [4,14,16]. Following successful engraftment, the recipient is at risk to develop several immune-related problems including graft rejection, graft-versushost disease (GVHD), post-transplant immunodeficiency, interstitial pneumonitis, and infectious complications (Table 1). Recurrent leukemia is an additional potential complication in leukemic recipients. Graft rejection probably results from histoincompatibility between donor and recipient and may be facilitated by immunization of the recipient via blood transfusions. In some instances defects in the marrow microenvironment may be responsible for graft failure [5]. Graft failure occurs in Table I. Areas of investigation 20-40 per cent of patients with aplastic anemia but is rare in patients with leukemia. This may relate to either inherent differences between the two diseases or to the more intensive conditioning used in leukemics. Storb and coworkers have reported a correlation between graft rejection and both recipient anti-donor immunity and marrow dose [15], and we have reported a correlation with pre-transplant lymphocytotoxins [6]. Transfusions also contribute to graft rejection. Graft-versus-host disease results from the introduction of immunocompetent donor cells into the immunosuppressed recipient. Principle target organs of GVHD include the lymphoid system, skin, liver, and gastrointestinal tract [17]. GVHD in man results from incomplete matching for nonHLA histocompatibility antigens. The loss of normal immune regulatory mechanisms and autoimmunity may also contribute. While GVHD initially results from immune stimulation, the end result is immunodeficiency. The incidence of GVHD following HLA-identical marrow transplantation is 70 per cent, and over one-half of these cases are fatal. The prevention and treatment of GVHD are problematic. Methotrexate is routinely given prophylactically to modify GVHD, but this is not completely effective. Attempts to prevent GVHD with antithymocyte globulin (A TG) or to treat it with A TG , corticosteroids, and other imm unosuppressive drugs have been largely unsuccessful. While complete histocompatibility matching would theoretically prevent GVHD, this approach would further limit the number of potential candidates for bone marrow transplantation. The removal of immunocompetent cells from the marrow inoculum prior to transplantation by either physical or immunologic technics has appeal but has not been critically evaluated in man. The complete prevention of GVHD is not necessarily desirable since GVHD may have anti-leukemic effects. Allogeneic marrow transplantation is followed by a period of immunodeficiency lasting several months to 1-2 years [7,20]. The cause of the immunodeficiency is multifactoral and includes abnormal or delayed lymphoid differentiation, GVHD, and the effects of immunosuppressive drugs. Posttransplant immunodeficiency is characterized by abnormalities of both T and B lymphocyte function including decreased antibody synthesis, decreased responsiveness to polyclonal mitogens, and inability to be sensitized to dinitrochlorobenzene (DNCB). Reactivity to alloantigens and skin graft rejection are normal. This immunodeficiency is correctable with time. This suggest that either a small number of lymphoid precursors are engrafted. or that their development is delayed. We have found no evidence of suppressor cells or factors in these patients [7]. Approximately 60-70 per cent of marrow graft recipients develop interstitial pneumonitis [ II ]. The incidence is higher in leukemic patients than in aplastics. One-half of cases are related to cytomegalovirus (CMV), 10 per cent to pneumocystis, and 10 per cent to other viruses. No etiology is identified in the remaining cases. It is likely that immunologic factors including immune stimulation, immunodeficiency, and GVHD playa critical role in the development of interstitial pneumonitis. Radiation and/or chemotherapy are probably not primary factors but may compromise resistance. In CMV pneumonitis, it is likely that both reactivation of latent endogenous infection and exogenous infection are important factors. Attempts to prevent or treat interstitial pneumonitis with antiviral chemotherapy (ara-A) have been unsuccessful. Studies of CMV immune globulin, or plasma and interferon, are currently underway at several centers. Bacterial and fungal infections are an important complication of bone marrow transplantation [19]. These usually occur during the period of granulocytopenia immediately following the transplant and their magnitude is related to the intensity of the conditioning regimen. Most patients receive oral non-absorbable antibiotics for gastrointestinal tract sterilization. systemic antibiotics, and granulocyte transfusions. The value of prophylactic granulocyte transfusions and laminar air flow environments is controversial, but recent data suggest they may decrease the incidence of infection without a substantial effect on survival [2].
The survival of patients with resistant acute leukemia is poor
with median survival of less than 6 months in several large series.
Because of this, we and others have studied the potential role of
allogeneic bone marrow transplantation in patients with resistant
disease. Transplantation in acute leukemia is difficult. In addition
to the previously described immunobiologic problems, it is necessary
to permanently eradicate the leukemic clone(s). A variety of chemotherapy-radiation
therapy regimens have been developed to achieve this goal. Three
representative regimens are indicated in Fig. I and remission and
survival data in Figs. 2 and 3 [8,9, 13, 18]. Several important
points emerge from these studies: I. Leukemic relapse is common
despite the use of supralethal levels of drugs or radiation; 2.
The risk of relapse is high during the first 2 years but lower thereafter;
3. That with the possible exception of SCAR! (see legend Fig. I),
more intensive conditioning has not been associated with a lower
relapse rate; and 4. 15-20 per cent of patients with resistant disease
may become long-term disease-free survivors. While this survival
rate is not a satisfactory end result. it is probably superior to
chemotherapy alone. It is noteworthy that immunologic problems rather
than resistant leukemia are the major cause of death in some series.
These problems may ultimately prove more soluble than resistant
leukemia. 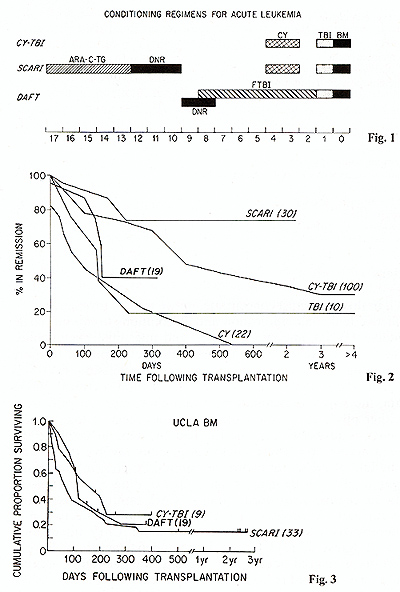
Future research in this field must concentrate on two critical
problems:
Bone marrow transplantation is an experimental approach to the treatment of patients with acute leukemia, aplastic anemia, and other neoplastic and genetic diseases. To date, long-term disease-free survival has been achieved in a small proportion of carefully selected patients with resistant acute leukemia. While results are not optimal, they are acceptable in late stage patients where there are no effective alternates. Major problems in marrow transplantation for leukemia include tumor resistance and a spectrum of immunologic complications including GVHD, immunodeficiency, and interstitial pneumonitis. Potential approaches to these problems have been suggested. Progress in anyone area would have a substantial impact on improving survival and extending the applicability of marrow transplantation to patients at an earlier stage of their disease.
I. Bach. F. H.. Van Rood. J.J.: The major histocompatibility complex.
N. Engl. J. Med. 295, 806-813: 872-878: 927-935 (1976) |