|
Heinrich-Pette-Institut für Experimentelle Virologie
und Immunologie an der Universität Hamburg
Retroviral vectors designed to transduce hemopoietic stem cells have to be optimised with respect to basic requirements for gene transfer and expression. (i) The cis-regulatory elements of the genomic vector RNA interacting with retroviral proteins supplied from packaging cells must allow for optimal packaging, reverse transcription and integration into the genome of the target cell. (ii) The cis-regulatory elements of the integrated 'proviral' vector DNA interacting with the cellular transcriptional machinery must guarantee efficient and long-lasting gene expression in the target cell and its progeny. Conventional vectors rely on cis-regulatory elements of the murine type C retrovirus Moloney murine leukemia virus (MoMuL V), and its relative, Moloney murine sarcoma virus (MoMuSV) [review in Miller, 1992a]. The cis-regualtory elements incorporated into retroviral vectors generally include the U3 regions of the long terminal repeat (L TR) and leader sequences. The U3-region displays the vector's enhancer/promotor setting, greatly contributing to tissue tropism of vector mediated gene expression [review in Stocking et al., 1993]. The leader can be referred to as the complete 5' untranslated part of the vector RNA that is necessary to fulfill the retrovirallife cycle. Hence, it contains the R and U 5 region of the L TR, the primer binding site (PBS) for the tRNA-primer initiating reverse transcription and the packaging region mediating efficient pseudotyping of the vector RNA in packaging cells. State of the art MoMuLV /MoMuSV -based vectors allow for efficient and safe production of high-titre stocks in safety-modified packaging cells of fibroblast origin [review in Miller, 1992a]. This has been achieved by inclusion of an extended leader containing gag-coding sequences that were altered to eliminate residual expression of viral protein [Bender et al., 1987; Armentano et al., 1987]. However, when targeting hemopoietic cells, MoMuL V -based vectors suffer from low gene expression levels in primitive stem cells and derived myeloid lineages [review in Stocking et al., 1993; Moritz and Williams, 1994]. This can be attributed to a lack oftransactivation from lymphotropic MoMuL V -type U3 regions as well as to transcriptional inhibition mediated by a yet unidentified repressor binding at the primer binding site (PES) of the retroviralleader [Eaum et al., submitted]. The seemingly stem cell specific block involving the PES for tRNAPro ofMoMuSV/MoMuLV can be relieved by usage of a PES for tRNAGln, derived from d1587rev, a recombined virus arosen from a defective MoMuL V and an endogenous murine retrovirus [Colicelli and Goff, 1987, Grez et al., 1990]. A leader fragment containing this permissive PES and downstream located sequences extending into the packaging region of the retroviralleader has been used to generate the murine embryonic stem cell virus (MESV), the first retroviral vector cloned mediating successfull gene transfer and expression in primitive embryonic stem cells (ES) [Grez et al., 1990]. Usage of the PES from d1587rev/MESV is also associated with ability of long-term gene expression from retroviral vectors in hemopoietic cells in vivo [Hawley et al., 1992; 1993]. Hence, MESV-based vectors have been suggested to be promising candidates for use in somatic gene therapy protocols involving retroviral gene transfer to hemopoietic cells [review in Stocking et al., 1993; Moritz and Williams, 1994]. Here, we provide evidence that sequences of MoMuSV/MoMuLV located in the retroviral packaging region also contain an inhibitory element blocking retroviral gene expression in a stage and lineage-specific manner within the hemopoietic system. Again, this block can be abolished by utilization of corresponding sequences of d1587rev/MESV. Thus, leadersequences formerly believed to be solely involved in interaction with viral proteins in vector packaging might also interact with cellular proteins contributing to vector transcription. However, sequence degeneration in the packaging region might contribute to slightly suboptimal packaging of MESV -based vectors.
Our MESV-based vectors contain leader sequences from d1587rev between
Kpnl (bp 37 of the leader) and Pst1 (bp 574) [sequence numbering
according to Colicelli and Goff, 1987]. The remaining vector backbone
is derived from PCMV (PCC4 embryonal carcinoma cell passaged myeloproliferative
sarcoma virus [Franz et al., 1986; Hilberg et al., 1987]). Leader
sequence comparison between the MESV -based vectors and MoMuL V
-based vectors reveals a number of point mutations, insertions and
deletions contributing to less than 80% homology at the DNA level
in the exchanged leader fragment (Fig 1 ). 5 point mutations convert
the PES for tRNAPro of MoMuLV/MoMuSV to the PBS for tRNAGln of d1587rev/MESV.
The PBS for tRNAPro overlaps in 17 of its 18 bp with the binding
site for the yet unidentified stem-cell specific repressor also
active in hemopoietic stem cells, therefore it was also referred
to as repressor binding site [Kempler et al., 1993]. Downstream
of the PBS follows the packaging region (). Sequences absolutely
necessary for packaging are located between Msc I (bp 216) and Xma3
(bp355). Removal of that fragment is sufficient to exclude packaging
of retroviral transcripts, a feature used for construction of safety-modified
packaging cells like GP&env86 or GP&env AM 12 [Markowitz et al.,
1988a; 1988b] .Within this fragment, a stretch of remarkably low
sequence conservation (less than 50% ) is observed, named nonhomology
region I [Collicelli and Goff, 1987]. Evidence obtained from analysis
of oncogenic avian retroviruses suggests that a 254-bp sequence
immediately downstream of the PBS, hence included in the exchanged
fragment and spanning the nonhomology region I, resembles an RNA
enhancer [Boerkoel and Kung, 1992]. Sequences downstream of Pstl
(bp 574) are identical between MESV -based vetors and MoMuLV /MoMuSV
-based vectors like LNL6 [Miller and Rosman, 1989] .Thus, MESV -based
vectors contain the extended leader including 418 bp 5' gag coding
region whose A UG (bp627) has been mutated to TAG to preclude translation
initiation. This region is required for optimal packaging [Bender
et al., 1987; Armentano et al., 1987]. We further studied the implications
of the sequence degeneration in the packaging region of MESV for
vector transfer and expression. 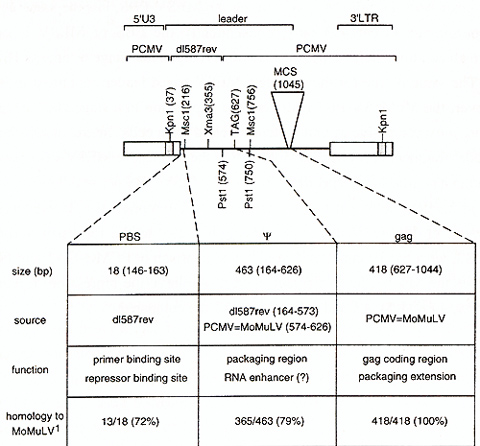
A second repressor function is located in the MoMuSV/MoMuLV-leader
downstream of the primer binding site,. in contrast, the corresponding
region of MESV is permissive. PCME-CAT (PCMV-LTR followed by MESV-PES
and truncated at Msc1 (bp 216 of the leader), originally named PCMV(EX)/BaI587-CAT)
and PCMO-CAT (PCMV-LTR followed by MoMuSV-PES and truncated at Msc1
(bp 216), originally named PCMV(EX)/Balp-CAT) have been described
previously [Grez et al., 1990]. In order to elucidate the influence
of the leader region located downstream of the PES for vector expression,
CA T -reporter gene vectors were cloned containing the U3 region
of PCMV followed by the complete leader of MESV (PCMEME-CA T) or
MoMuSV /MoMuL V (PCMOMO-CA T), respectively. To seperate the influence
of downstream sequences from that of the PES, CAT -vectors were
cloned containing the MESV -PES followed by the complete downstream
leader sequences of MoMuSV /MoMuL V (PCMEMO-CA T) and, vice versa,
the PES of MoMuSV followed by downstream leader sequences ofMESV
(PCMOME-CAT). This was achieved by exchanging a 560 bp Msc 1 fragment
of the MESV leader with the corresponding 528 bp fragment of V -MDR
(a MoMuLV/MoMuSV based vector kindly provided by A. Deisseroth,
MD Anderson Cancer Center, Houston, Texas). These vectors were transfected
into the pluripotent murine myeloid stem cell line FDC-Pmix 15S
and granulocyte/macrophage precursor line FDC-P1, the human T -lymphoblast
CCRF-CEM as well as in the murine fibroblast NIH3T3 cells as previously
described [Eaum et al., 1994]. The results are shown in Fig.2. As
reported elsewhere, PCME-CA T , containing the MESV -PES, has the
same activity as a construct bearing the PCMV-U3 only, consequently
the PES of MESV is completely permissive in all cell lines studied,
irrespective of developmental stage or lineage [Eaum et al., submitted].
The same is true for the complete MESV-based leader contained in
PCMEMECAT. However, the MoMuSV-PES restricts gene expression in
a stage and lineage specific manner, being most repressive in multipotent
myeloid stem cells. Interestingly, the fusion of the Msc l-leader
fragment of MoMuSV /MoMuL V to the permissive MESV -PES restricts
CAT accumulation in a similar tissue-specificity as the MoMuSV -PES
per se: silencing activity can be detected in myeloid cells, but
not in fibroblasts or T-lymphoblasts. However, the Msc1leader fragment
of MoMuSV does not add significantly to the repressor activity of
the MoMuSV -PES, what can be concluded from the comparison of PCMO-CA
T and PCMOMOCAT; nor does the Msc1-leader fragment of MESV relieve
the repression triggered by the MoMuSV-PES, ifPCMO-CAT and PCMOME-CAT
are compared. 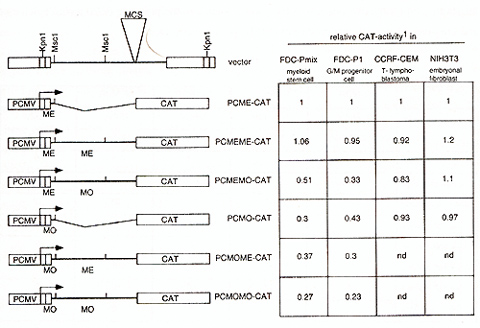 Fig.2 : Influence of retroviralleader on gene expression in different developmentallineages. 1CA T -activity was determined with the CAT -Elisa Kit (Boehringer Mannheim), and corrected for transfection efficiency measured by cotransfected galactosidase-activity. Values shown represent mean values of at least two independent transfections and are given as ratio to PCMECAT, whose activity was set as 1. ME, sequence derived from MESV. MO, sequence derived from MoMliSV /MoMuLV. Influence of the MESV-leader on packaging. Taking into consideration the relatively low degree of conservation in the leader sequences necessary for packaging of the vector's genomic RNA, the feasibility of the MESV -leader to obtain high-titre producer clones has to be investigated. We therefore established large numbers of amphotropic producer clones for MESV -based retroviral vectors via retroviral infection of amphotropic packaging cells GP&envAM12 with supernatants from ecotropic producers. The vectors transduce the dominant selectable marker human multiple drug resistance 1 (hmdrl) [review in Gottesman and Pastan, 1993]. The basic vector architecture is comparableie 5'U3-leader-hmdr1-3'LTR. In the MESV based vectors, the LTRs are of PCMV, myeloproliferative sarcoma virus (MPSV), Harvey murine sarcoma virus (HaMliSV) or spleen focus forming virus (SFFVp) origin [Baum et al., submitted]. In comparison to the MoMuL V /MoMliSV -based V -MDR 1, the MESV -based vectors consistently yield slightly lower titres or lower incidence of high-titre clones (Table I). Titres were determined by infection of ecotropic fibroblast packaging cells GP&env86 with supernatants from amphotropic producer clones and subsequent selection in the presence of 20 µg/ml colchicine and are given as mdr l-transduced units (MTU) per ml supernatant. 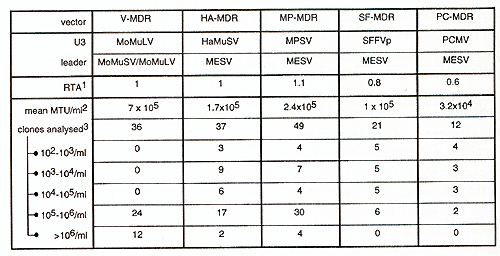

Murine retroviruses and derived retroviral vectors have been used for a number of years to study developmental properties of hemopoietic cells. More recently, the boom in somatic gene therapy studies contributed to an enhanced demand for retroviral vectors that are both safe and highly efficient for long-term gene expression in lymphoid or myeloid cell. However, the efficiency of retroviral gene expression obtained by conventional MoMuL V -based vectors is quite low. Insufficient transactivation as well as progredient extinction of the activity of retroviral cis-regulatory elements seems to compromise their use in a variety of approaches relying on high levels and long-term maintenance of gene expression [reviews in Miller, 1992b; Stocking et al., 1993; Moritz and Williams, 1994]. Since the level as well as the duration of vector-mediated gene expression is determined by interactions of the vector's cis-elements with trans-acting proteins of the host cell, a systematic analysis of the molecular prerequisites for efficient vector function is needed. In this report, we focus on the impact of the retroviralleader on vector function. A negativeacting element triggered by the PES of MoMuL V /MoMuSV has already been reported that inhibits high levels as well as long-term maintenance of vector expression in myeloid cells [Hawley et al., 1992; 1993; Eaum et al., submitted]. A similar seemingly stem cell specific repressor function of the MoMuL V /MoMuSV PES has been noticed for murine embryonal carcinoma cells and ES cells [Weiher et al., 1987; Grez et al., 1990]. In this study, we obtained evidence hat sequences located downstream of the PES in the putative packaging region of the leader also contain cis-acting elements with inhibitory function in myeloid cells. In contrast, both the PES and the packaging region of MESV are completely permissive for U3-driven gene expression in myeloid cells. Again, similar observations have been made in ES cells, resulting in usage of a leader fragment containing both the PES and the packaging region of the endogenous retrovirus d1587rev to assemble MESV [Grez et al., 1990]. The influence of the leader could be at the level of transcription or post -transcriptionally (RNA secondary structure, RNA half life, translation). The remarkable tissue specificity of the repressory effect does not rule out one of these possibilities. Interestingly, sequences located in a 254 bp fragment of the retroviralleader located immediately downstream of the PES might be involved in a positive-feedback activation of the 5'L TR and consequent downregulation of the 3 'L TR, as was suggested from studies in avian retroviruses. The mechanism might resemble that of an RNA enhancer, in that it is dependend on the position as well as the orientation in respect to the 5'LTR [Boerkoel and Kung, 1992]. Interestingly, in the corresponding region of MESV, the greatest degree of sequence degeneration is observed (nonhomology region 1). A more precise analysis of the repressor element(s) is desirable to elucidate the nature of the repressor as well as to design optimised leaders for retroviral vectors. The latter task has to take into consideration the role of the leader region in packaging of genomic vector RNA. The sequences necessary for packaging have initially been mapped in a small fragment of the MoMuL V -leader located immediately downstream of the retroviral splice donor [Mann et al., 1983]. Hence they were excluded from gag/pol and env -expression constructs incorporated into safety-modified packaging cells [Danos and Mulligan, 1986; Markowitz et al., 1989]. Later studies emphasized the necessity of afar extended leader extending into the 5' portion of the gag-coding region to obtain high-titre packaging [Bender et al., 1987; Armentano et al., 1987]. Vectors containing these sequences to optimize packaging are addressed as gag+vectors [Bender et al., 1987] .MESV -vectors are identical to MoMuL V /MoMuSV -vectors in regard to gag+, however, both the fragment absolutely necessary for packaging and the subsequent packaging region are less than 80% conserved in comparison to MoMuLV /MoMuSV -vectors. This could account for a slightly suboptimal packaging, a possible explanation for our observations that the first generation of MESVvectors does not as routinely yield high-titre producer clones as conventional MoMuL V /MoMuSV vectors do. On the other hand, degeneration of vector-leader sequences in comparison to corresponding sequences contained in transgenes of packaging cell lines makes sense for safety reasons, because it reduces the possibility of homologous recombination necessary to create replication competent viruses out of replication-incompetent packaging cell transgenes. Therefore, dissection of the leader's cis-elements involved in packaging and transcription is an important prerequisite to develop fully permissive retroviral vectors for gene therapy applications.
This work was supported by the Deutsche Krebshilfe. The Heinrich-Pette-Institut
is financially supported by Freie und Hansestadt Hamburg
Armentano D, Y u SF, Kantoff PW, von Ruden T, Anderson WF and Gilboa
E ( 1987) Effect of internal viral sequences on the utility of retroviral
vectors. J. Virol., 61: 1647-1650 |