|
|
|
In experimental models, the purging with anti-B cell monoclonal
antibodies (MoAb) and complement has been shown to eliminate at
least 3-4 log of Burkitt cells [1,2] .The same procedure has been
used for patients with B cell lymphoma entered in an autologous
bone marrow transplantation (ABMT) protocol [3-5]. We review here
47 cases of ABMTwhose autograft has been purged with monoclonal
antibodies and complement (MoAb + C') and analyze the toxicity of
the procedure on progenitor cells, as well as the hematological
and immunological recovery after grafting. In 29 patients pathological
BM was observed at least once during the course of their disease
, and 10 had detectable BM micrometastases on the day of harvest,
thus the usefulness of the purging procedure ist also discussed. Materials and Methods Patients Table 1. Clinical characteristics
of the patients (n = 47) 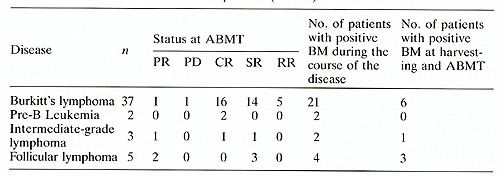
BM harvest and purging procedures have been also described previously [ 1-31. Briefly, after ficoll separation mononuclear (MN) BM cells were resuspended in Hanks solution at 2 times 10 high 7 cells/mI with calcium and magnesium and incubated for 20 min at 24°C with MoAbs (see below) at appropriate dilution and washed. Samples were then treated twice with baby rabbit C' (Pasteur, Lyon, France) for 60 min at room temperature, with one wash between the two treatments; the C' was used at 1/3 final dilution. DNAse (2.5 U/ml) was added during the two courses of C' incubation. Finally, cells were resuspended in the freezing solution. Y29/55, a pan-B non clustered MoAb, was kindly provided by Dr. Forster; AL2, a CD10 MoAb (anti-CALLA), was provided by A.M. Lebacq, and RFB7, a CD20-like pan-E MoAb, was supplied by G. Janossy. Finally SB4, a CD19 MoAb, was given by J.C. Laurent (Sanofi, Montpellier, France ). The BM was treated with Y29/55 alone in 7 cases, with Y29/55 + AL2 in 6 cases, with Y29/55 + RFB7 + AL2 in 16 cases and with RFB7 + SB4 in 18 cases. The toxicity of this procedure was evaluated by the clonogeneic efficiency of the CFU.GM progenitors in agar.
The T cell ratio and percentage of NK cells were evaluated by immunofluorescence (IF) analysis on a microscope. The proliferative index of T cells after 5 days of PHA stimulation at 0.5 µg/ml was measured by thymidine incorporation. IL2 secretion by PEL after 72 h of stimulation with PHA was measured by the capacity of the supernatant to induce the proliferation of the IL2-dependent cell line, CTL2, compared to a standard medium. NK function was measured in a 51Cr release assay on K562 cell line ( 40: 1 ratio).
Patients had 2-4 trephine biopsies and 2-4 aspirates at diagnosis, relapse, and on the day of harvest. After Ficoll separation of the BM cells for the purging procedure, 2 times 106 cells were analyzed with a single IF method using 2 pan-B MoAbs RFB7 and SB4. In Burkitt's lymphoma, cells were cultured in a liquid assay, as previously described [6,7 ,91. Malignant cells are detectable from day 8 of culture by cytology and the establishment of a cell line allows to confirm the positivity of the BM samples as previously shown in detail [6, 7].
As shown in Table 2, the purging procedure was not toxic for progenitor cells when assayed for their clonogenic efficiency (CFU-GM/2 times 105 cells). Nevertheless, a significant loss of MN cells was seen and both the recovery of total MN cells/kg and CFU-GM/kg was about 25% of the harvest. In terms of toxicity, no difference was observed between the four combinations of MoAbs used for the purging procedure. After reinfusion into patients, their hematological recovery was quick: the median time to reach 0.5 times 109 granulocytes/1 was 24 days (range 10-42 days), and the median time to reach 50 times 10 high 9 platelets/1 was 27 days (range 8-60 days). Only one patient had delay to engraftment and he presented with an excess ofCD8+ , leu7+ cells in the blood and the BM. This patient was then treated for 6 days with intravenous injections of CD8 MoAb (provided by A. Bernard) at 0.2 mg/kg per day [8] .On CD8 treatment in vivo, the CD8+ , leu7 + circulating cells disappeared from the circulation, and both granulocytes and platelets recovered within 2 weeks after the start of injections. The immunological recovery is summarized inTable 3. Before the graft the Tcell functions, evaluated by the number ofT4 and T8 cells, the proliferative response to PHA and IL2 secretion were subnormal, as was the NK function. The B cell function, assessed by the level of immunoglobulin secretion, was subnormal (serum IgG levels in the normal range, IgM and IgA levels at the lower limits of the normal range). After grafting the patients had T cell deficiency for up to 1 year and there were variations between patients. The recovery of normal Tcell functions did not correlate with a lower incidence of relapse.The NK function was maximal between days 60 and 90 but decreased Table 2. Total BM Mononuclear cells and
CFU-GM harvested and reinjected in MoABs and complement purged BM
in B cell lymphoma 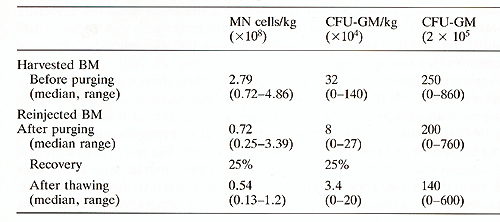
Table 3. T, NK, and BCell Functions with MoABs and complement purged autograft in Bcell lymphoma 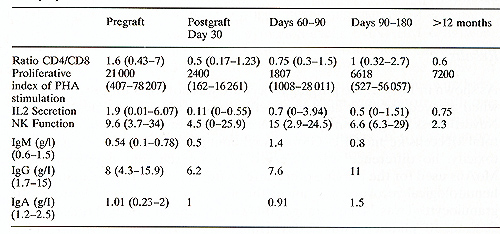
Table 4. Clinical Characteristics of
Patients with positivc BM at time of harvesting 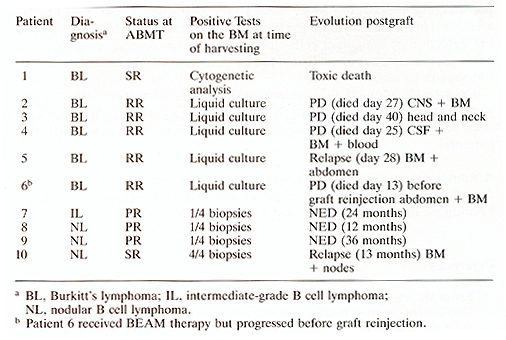
The purging procedure with anti-E MoAbs and complement in E cell lymphoma is harmless in itself. The hematological reconstitution is rapid. As already observed after AEMT [10] , patients had a prolonged and severe Tcell defect after AEMT. Interestingly, E cell functions were normal within 60 days post-grafting despite the purge of EM with pan-E monoclonal antibodies. NK functions were soon normalized after grafting and showed high values for 90 days then decreased after 3 months. However, the usefulness of purging procedures is still debated. In this series of 47 patients we have clearly demonstrated that the purging was efficient for 5 patients with Eurkitt lymphoma, and in spite of this [9] the patients were refractory to conventional therapy and relapsed postgrafting due to the failure of high-dose chemotherapy to control their disease. For this reason, patients with EM involvement should not be considered for such therapeutic protocols in Eurkitt lymphoma. In the other E cell lymphomas the methods of detection of malignant cells used here are not accurate enough to permit the quantification of malignant E cells in the autograft. The purging was justified in the four patients with EM micrometastases on the day of EM harvest but is was not possible to correlate the favourable outcome after ABMT to the efficiency of the purging procedure. More powerful conclusions could possibly be addressed in follicular lymphoma by the use of polymerase chain reaction for the detection of malignant cells characterized by molecular abnormalities, although these results are still debated by other groups (see other chapters in this volume). The attitude adopted in our group for the treatment of B cell lymphoma is to reserve purging procedures to patients who had a pathological BM at least once during the course of the disease (27 out of 48 in this series of high-risk patients).
|