|
Terry Fox Laboratory and the Leukemia/Bone Marrow
Transplantation Program of British Columbia, British Columbia Cancer
Agency,
Vancouver General Hospital and the University of British Columbia,
Vancouver, B.C.
Long-term maintenance of normal hematopoiesis in vitro is possible
when very primitive progenitors are cocultivated with certain non-hematopoietic
stromal cells that may co-exist in (or be derived from cells that
co-exist in) hematopoietic tissues. Such long-term cultures (L TC)
have been used to develop quantitative assays for the most primitive
populations of hematopoietic cells currently detectable in adult
marrow. In addition they provide a unique model for analysis of
the complex molecular mechanisms that may regulate primitive hematopoietic
cell population dynamics in vivo. Similar studies with L TC of cells
from patients with chronic myeloid leukemia (CML) have made it possible
to detect and characterize very primitive neoplastic cell populations
in this disease. These latter studies have revealed differences
in the properties of primitive CML cells that both reflect and explain
their increased turnover and are thus presumably part of the mechanism
that enables the neoplastic clone to expand in vivo. In addition,
the most primitive neoplastic cells in CML patients are abnormally
distributed between the marrow and blood and their ability to maintain
their numbers in L TC has also been found to be defective. Assessment
of the number and behaviour of primitive cells in L TC of CML marrow
has been used to identify those patients most likely to benefit
from intensive therapy supported by transplantation of cultured
autologous marrow. Twenty-two such CML patients have now been treated
with this experimental protocol. The results to date have clearly
established the feasibility of this novel treatment strategy and,
together with more recent laboratory findings, suggest future avenues
for significantly improving the management of CML patients.
INTRODUCTION
Perturbations of the norm frequently provide an opportunity to
gain new insights into poorly understood regulatory processes. Where
the perturbation has a known genetic basis, this information may
serve as a unique clue to the delineation of the underlying molecular
mechanism(s) affected. Chronic myeloid leukemia (CML) is a prime
example of a disease of perturbed hematopoiesis associated with
a consistent and unique genetic abnormality characterized at the
molecular level as the creation of a fusion gene involving BCR and
ABL.l CML is also of considerable clinical importance as a disease
entity since it is inevitably fatal in its acute phase. At the cellular
level, CML is recognized to be a clonal, multilineage myeloproliferative
disorder arising from the dereglilated proliferation of a pluripotent
hematopoictic stem cell and its subsequently amplified progeny'
which, by the time of diagnosis, have typically come to dominate
the entire hematopoietic sy.stcm.▓ Initially, differentiation processes
appear to be relatively' unaffcctcd. Thus early expansion of the
CML clone leads to the continued generation of functionally normal
mature blood cells altlough the rate of granulocyte and macrophage
production may. be increased more than 5O-fold However, on average
within 4 years of diagnosis, evolution from this chronic phase to
a frank acute leukemia occurs, This change is typically characterized
at the genetic level by the acquisition of additional mutations
and phenotypically by a breakdown in differentiation leading to
a rapid accumulation of non-functional blasts.│ Recent studies of
the molecular and cell biology of CML have led to abetter understanding
of both norm1al and leukemic hematopoietic stem cell regulation
in addition to stimulating the development of new approaches to
treatment. This review summarizes some of the progress in these
areas that has emerged from a combined laboratory and clinical effort
at our centre to improve the management of patients ,with CML using
intensive therapy supported by autologous marrow rescue The multilineage
nature and likely pluripotent stem cell origin of CML was first
suggested by Dameshek long before direct evidence of a normal pluripotent
hematopoictic stem cell compartment was obtained.4 This came almost
simultaneously about a decade later from two independent lines of
,work. One involved documentation of the multi-lineage differentiation
potential of single cell-derived clones generated in the spleens
of mice transplanted with small numbers of adult mouse bone marrow
cclls5 The second was the recognition of a consistent chromosomal
abnormality (the Ph chromosome) in virtually all dividing marrow
cells of CML patients, including erythroid cells and megakaryoeytcs6.7
Formal proof that these Ph chromosome-positive cells represented
expanded clonal populations was subsequently provided following
the development of methodology for analyzing the activity of X-Iinked
genes in hematopoietic cells from hetcrozygous females ,with CML▓
These types ofapproaches have now been extended to show that the
neoplastic clone may also conmmonly include Band T lymphocytes.
as well as members of all of the mycloid lincages.8-10 With the
introduction of reproducible in vitro assays for quantitating human
progenitors on different hematopoietic pathways and at different
stages of differentiation, II and the development of procedures
for obtaining cytogenetic data from individual colonies to distinguish
their leukemic or norm1al origin,12 considerable information about
early leukemic cell compartments has accrued rapidly. For example,
it is now well established that, at the level of cells detectable
as in vitro colony'-form1ing cells, Ieukemic progenitor numbers
on all myeloid lineages appear, on average, to be expanded equally
relative to one another and their cycling control is also deregulated
in a lineage non-specific fashion. These findings are of interest
since, prior to initiation of treatment, platelet counts are usually
increased to a much lesser extent that the WBC count and the hematocrit
is frequently lower than normal (reviewed in Ref 13). These findings
imply that the BCR-ABL gene is active at early. stages of hematopoietic
cell differentiation, but in a fashion that this stage does not
discriminate between lineages nor affect early' commitment events
per se. Ho,w an ovcrproduction of all ty'pcs of early progenitors
is translated in vivo into an overproduction of mature cells, to
a large extent only on the granulopoietic pathway. is not known.
Nevertheless, the large numbers of early types of Ph-positive cells
on all lineages explains ,why, it is usually. difficult to detect
any' norm1al hematopoietic cells 1ntercstingly. analysis of progenitors
from newly diagnosed CML patients with small tumor burdens has shown
that normal numbers of normal progenitors are present.14 Thus it
could be inferred, as was eventually shown, that even more primitive
types of norm1al hematopoietic cells are also likely to be present
in substantial numbers in many CML patients. 15,16
RESULTS AND DISCUSSION
Use of the Long-term Culture (L TC) System to Analyze Early Events
in Normal and Leukemic Hematopoiesis.
Three features of hematopoiesis in marrow L TC have allowed the
unique application of this system to the study of early stages of
hematopoiesis. The first is the fact that the system is a dynamic
one in which cell proliferation, differentiation and death occur
continuously for many weeks.17 Thus, eventually, all of the differentiated
hematopoietic cells present should be derived from a very primitive
cell type present in the original input suspension. Moreover, if
the supportive "stromal " elements required (which are derived from
precursors of the fibroblast-adipocyte-endothelial lineages), are
provided independently in non-limiting numbers, then after an appropriate
interval, the number of differentiated hematopoietic cells present
might be expected to be quantitatively related to an input population
of very! primitive hematopoietic precursors. In the case of human
cells, the number of in vitro colony-forming cells present in L
TC initiated under these conditions and assessed after a minimum
interval of 5 weeks has been found to be a suitable endpoint for
the measurement of an input cell type with properties shared exclusively
with long-term in vivo repopulating cells.18,19 Because of the assay.
procedure used for their detection and quantitation, these cells
are referred to as LTC-initiating cells or L TC-IC. We have shown
that the relationship between input L TC-IC and their 5 week clonogenic
cell output is linear down to limiting numbers of L TC-IC seeded
into the assay cultures. It is therefore possible to use limiting
dilution analysis techniques to derive absolute frequencies of L
TC-IC and hence to ascertain the output characteristics of individual
LTC-IC.18,20 The second feature of the L TC system is that the more
primitive types of hematopoietic cells localize within and tend
to remain in the adherent layer. This latter fraction of the culture
also contains the stromal cells essential for L TC-IC support,21,22
Nevertheless, once the adherent layer is established, the majority
of the primitive hematopoietic cells in it are normally maintained
in a quiescent state unless the cultures are perturbed by' the addition
of specific factors (or fresh horse serum) that activate fibroblasts
(and endothelial cells). This leads to the activation of primitive
hematopoietic progenitors which is then follow\'ed by' their spontaneous
return to a non-cycling state a few days later unless the cultures
are again perturbed. The ability to up- and down-regulate primitive
progenitor cycling in this way can be repeated for many weeks23
The LTC system has thus provided a convenient model for identifying
both stimulating and inhibitory. factors that may be involved in
the stromal cell-mediated control of primitive hematopoietic progenitor
proliferation.24 A third important feature of the L TC system is
its ability' to support not only the production of mature cells
from very primitive hematopoietic precursors (L TC-IC) but also
the self-renewwal and maintenance of cells with in vivo repopulating
potential.25,26 Thus the L TC system should also prove useful for
identifying molecular species that regulate this key stem cell function,
Assessment of the behaviour of CML cells in L TC has allowed delineation
of a number of aspects of early progenitor cell behaviour that are,
or are not, perturbed by the neoplastic process, These are summarized
in Table I, Of note was the finding early on that the deregulated
cycling activity previously documented for primitive Ph-positive
colony-forming cells in vivo is reproduced in the L TC system.21
Subsequent studies failed to detect evidence of an underlying autocrine
or paracrine mechanism to explain the abnormal cycling behaviour
of primitive CML cells both in vivo and in vitro,27 Additional experiments
suggested that these cells are normal in their responsiveness to
the growth inhibitory' effects of TGF -▀, even under varying conditions
of stimulation28 These unexpected findings posed a significant challenge
to current models of the molecular mechanisms thought to control
early hematopoietic cell turnover and led to the concept of co-operative
inhibition, i,e", a mechanism in which the effects of two inhibitors
might be additive or even synergistic, Thus one might envisage an
additive effect between TGF-▀ and (an)other endogenous inhibitor(s)
in the L TC system, neither of which at the levels prevailing in
unperturbed L TC, would alone be sufficient to arrest the cycling
of primitive normal cells. According to such a model, insensitivity
of CML cells to only one component of this co-operating inhibitory'
mechanism would then give the observed picture of deregulated cycling
Table 1. Properties of Primitive Ph-positive Populations
as Revealed by L TC Studies.
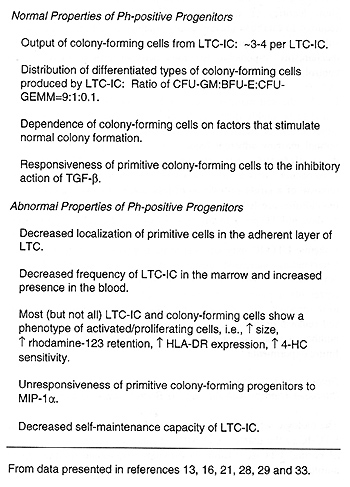
Interestingly, the results of initial studies of the role of MIP-I
alfa in the LTC system bear out the predictions of such a model
of co-operative inhibition.29 Whether MIP-I alfa ( or other . similarly-acting,
factors) normally plays a co-operating inhibitory role in vivo is,
of course, still a matter of speculation. However, some support
for this possibility has recently been provided by studies in .mice
demonstrating predicted effects of appropriately timed administration
of MIP-alfa on primitive hematopoietic cell sensitivities to cycle-active
drugs.30,31 From the point of view of understanding CML, the hypothesis
that MIP-I a is a physiologically relevant negative regulator of
primitive normal cells (to which CML cells do not respond) is particularly
attractive because it offers an explanation for the deregulation
of primitive leukemic cell turnover that is seen in CML patients.
Further analysis of how primitive CML cells are able to circumvent
MIP-1alfa effects may thus help to pinpoint the molecular mechanisms
leading to clonal dominance and hence possibly. to disease progression.
Two other points listed on Table I also deserve further comment.
One pertains to evidence of a deregulation of proliferation control
that is seen in CML at the level of the most primitive neoplastic
cells detectable, .i.e., leukemic L TC-IC. It is not difficult to
imagine that alterations in mechanism(s) thought to restrict the
turnover of primitive normal colony-forming cells might also apply.
to leukemic L TC-IC. On the other hand, stage-specific mediators
of positive (stimulatory) effects are well documented.22,32 It is
thus conceivable that a similar principle might apply to the target
cell specificity of different inhibitory. cytokines Also noteworthy
is the fact that a substantial proportion of primitive leukemic
cells do not show features of activated cells,33 Additional studies
will be required to establish unequivocally \whether or not these
represent a subpopulation of quiescent primitive leukemic cells
and. if so. the mechanisms responsible for such heterogeneity in
their c\.cling control. Finally, the self-maintenance of CML L TC-IC
in L TC has been found to be highly defective by comparison to normal
L TC-IC of either blood or marrow origin, even when these are cultured
on normal marrow adherent feeder layers,16 At present there is no
direct information as to why this rapid decline of CML L TC-IC in
vitro occurs. It is clearly not a technical artifact due simply
to the removal of a larger proportion of less adherent leukemic
cells when the cultures are fed, as the effect is most dramatic
during the first 10 days in L TC prior to any manipulation of the
cells It is inviting to speculate that the defective self-maintenance
exhibited by leukemic L TC-IC may, at least in part, be secondary
to a previous longstanding increased turnover rate of the leukemic
L TC-IC population in vivo. However. another possibility is that
this represents a more direct and immediate action of the BCR-ABL
gene product on intracellular pathways that regulate L TC-IC self-renewal
probabilities. Since these alternatives are testable by a number
of strategies, it should be possible to resolve this issue in future
experiments .
Potential of L TC to Improve the Treatment of CML Patients Using
Intensive Therapy with Autologous Bone Marrow Rescue
The biologic selection in vivo against the accumulation of leukemic
L TC-IC in the marrow of CML patients \with preferential retention
of normal L TC-IC (Table I) provides a rationale for the use of
autologous marrow transplants to allow the administration of potentially
curative myeloablative treatment regimens. In addition to this naturally
occurring benefit can be added a number of in vitro selection strategies.
Unfortunately, those that exploit differences likely to be related
to an altered proliferative status of primitive leukemic cells,
e.g., increased expression of HLA-OR, higher forward light scattering
characteristics, increased retention of rhodamine, and increased
sensitivity to 4-hydroperoxycclophosphamide ( 4-HC), also have such
a small selective potential that their clinical usefulness seems
dubious.33 At present, the most significant in vitro purging effect
has been obtained by incubating CML marrow under LTC conditions
for 10 days.16 Under these conditions leukemic L TC-IC numbers drop
30-fold whereas normal L TC-IC remain at input levels. As a result,
for patients whose initial marrows already contain readily detectable
frequencies of normal L TC-IC (>2% of normal values) and relatively
fewer leukemic L TC-IC, a theoretically attractive autograft. can
be obtained by incubating the marrow in LTC for 10 days prior to
transplantation In Vancouver, 22 CML patients found to fit into
such a group have been subsequently treated \with intensive therapy
and transplantation of a cultured marrow autograft. The most dramatic
finding in these patients has been the consistent recovery of normal
hematopoiesis within the first 2-7 weeks \with a fe\w exceptions
(6 patients). usually (5 patients), because the graft appeared to
have been inadequate. Of the 15 patients \who were transplanted
\with cultured marrow in first chronic phase, 13 are alive and 8
are in hematological remission up to 5 years later In the remaining
7 patients who were transplanted \with more advanced disease survival,
although poorer (3 alive), is still encouraging However. late reappearance
of some Ph-positive cells has also been a consistent, albeit late,
finding and additional strategies appear required to eliminate these
cells 11in this regard. the use of interferon post-autografting
seems to offer some potential. Clearly. more work remains to refine
and evaluate the role of culture purging in the development of a
curative treatment. The present findings do, however, underscore
the principle of developing new therapies based on careful scientific
investigations and highlight the possibility of new avenues on the
horizon for significantly improving the management of C M L patients
Acknowledgments.
The work described in this review was supported in part by the National
Cancer Institute of. Canada (NCIC) and the British Columbia Health
Research Foundation. C.J Eaves is a Terry Fox Research Scientist
of the NCIC. We also thank H, Calladine for manuscript preparation
and the numerous clinical and support staff of the British Colombia
Cancer Agency and Vancouver General Hospital who assisted in patient
care and data collection
REFERENCES
1. Groffen J, Heisterkamp N In. Goldman JM. Bailliere's Clinical
Haematology'. Chronic myeloid leukaemia.
Voi I London; Bailliere Tindall, 1987; p. 983.
2.Raskind WH, Fialkow PJ. Adv Cancer Res 1987:49127
3. Hagemeijer A. In. Goldman JM. Bailliere's Clinical Haematology.
Chronic myeloid Ieukaemia Vol I. London: Bailliere Tindall, 1987;
p. 963.
4.Dameshek W. Blood 1951 :6372.
5.Till JE, McCulloch EA Radiat Res 1961;14.213
6.Tough IM, Jacobs PA, Brown WMC, Baikie AG, Williamson ERD. Lancet
1963; 1 :844.
7.Whang J, Frei III E, Tjio JH, Carbone PP, Brecher G. Blood 1963;22:664.
8.Bernheim A, Berger R, PreudHonmme JL, Labaume S, Bussel A, Barot-Ciorbaru
R. Leuk Res 1981;5:331.
9.Martin PJ, Najfeld V, Hansen JA, Penfold GK, Jacobson RJ, Fialkow
PJ. Nature 1980;287:49.
10. Jonas D, Lubbert M, Kawasaki ES et al. 1992;79:1017.
11. Suther1and HJ, Eaves AC, Eaves CJ. In: Gee AP. Bone Marrow Processing
and Purging: A Practical Guide.
Boca Raton; CRC Press Inc, 1991; p. 155.
12. Fraser C, Eaves CJ, Kalousek DK. Cancer Genet Cytogenet 1987;24:
1.
13. Eaves CJ, Eaves AC. In: Goldman JM. Bailliere's Clinical Haematology.
Chronic Myeloid Leukaemia.
Vol 1. London; Bailliere Tindall, 1987; p. 931.
14. Kalousek DK, Eaves CJ, Eaves AC. 1984;3:439.
15. Coulombel L, Kalousek DK, Eaves CJ, Gupta CM, Eaves AC. N Engl
J Med 1983;308: 1493.
16. Udomsakdi C, Eaves CJ, Swolin B, Reid DS, Barnett MJ, Eaves
AC. Proc Natl Acad Sci US A 1992;89:6192.
17. Eaves CJ, Cashman JD, Eaves AC. J Tissue Culture Methods 1991;
13:55.
18. Sutherland HJ, Lansdorp PM, Henkelman DH, Eaves AC, Eaves CJ.
Proc Natl Acad Sci US A 1990;87.3584.
19. Eaves CJ, Sutherland HJ, Udomsakdi C et al. Blood Cells 1992;18:301.
20. Udomsakdi C, Lansdorp PM, Hogge DE, Reid DS, Eaves AC, Eaves
CJ. Blood 1992;80:2513.
21. Eaves AC, Cashm11an JD, Gaboury. LA, Kalousek DK, Eaves CJ.
Proc Natl Acad Sci US A 1986;83:5306.
22. Sutherland HJ, Eaves CJ, Lansdorp PM, Thacker JD, Hogge DE.
Blood 1991;78:666.
23. Cashman J, Eaves AC, Eaves CJ. Blood 1985;66.1002.
24. Eaves CJ, Cashman JD, Sutherland HJ et al. Ann N Y Acad Sei
1991;628:298.
25. Bamett MJ, Eaves CJ, Phillips GL et al. Transplant 1989;4:345.
26. Fraser CC, Szilvassy SJ, Eaves CJ, Humphries RK Proc Natl Acad
Sci US A 1992;89:1968.
27. Otsuka T, Eaves CJ, Humphries RK, Hogge DE, Eaves AC. Leukemia
1991;5:861.
28. Cashman JD, Eaves AC, Eaves CJ. Leukemia 1992~6.886.
29. Eaves CJ, Cashman JD, Wolpe SD, Eaves AC Blood 1992;80 Suppll:155a.
30. Dunlop DJ, Wright EG, Lorimore Set al. 1992;79:2221.
31. Lord BI, Dexter TM, Clements JM, Hunter MA, Gearing AJH. Blood
1992;79:2605.
32. Leary AG, Zeng HQ, Clark SC, Ogawa M. Proc Natl Acad Sci US
A 1992;89:4013.
33. Udomsakdi C, Eaves CJ, Lansdorp PM, Eaves AC. Blood 1992;80:2522.
|
