|
Department of Internal Medicine, Division of Hematology and Oncology, Karl-Marx-University, 7010 Leipzig, German Democratic Republic A. Introduction The development of suitable methods for the purging of malignant bone marrow contaminating cells in patients with acute leukemia may offer a better chance of success for autologous bone marrow transplantation. In addition to investigating the wanted effect, i. e., damage to leukemic cells, it is important to investigate the tolerance of normal hematopoietic stem cells within these manipulations in order to guarantee the grafting of the purged transplants. Because yp 16-213 is discussed as a potent agent for eliminating tumor cells in vitro [1,2], we incubated bone marrow with this drug. Our aims were to determine what doses of yp 16- 213 are tolera ted by normal hematopoietic stem cells, and whether there is a differenee between the behavior ofGM-CFC and LTBMC stem cells after drug incubation.
I. Drug Incubation
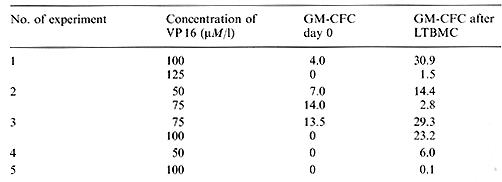
At initiation of all LTBMCs an aliquot of the sample was routinely
tested for GMCFC. The effects of yp 16-213 incubation on GM-CFC
are shown in Fig. 1. It is obvious that all doses tested had a strong
cytotoxic effect. Considering the mean values of recovery, the cytotoxic
effect was more pronounced in bone 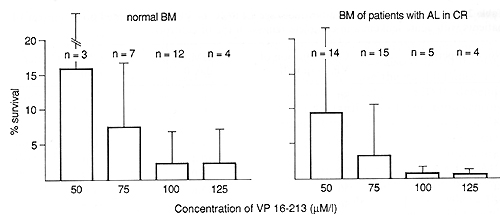
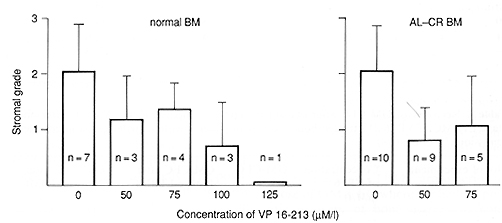 Fig.2. Degree of adherent layer establishment in 3-week-old LTBMCs of VP 16-213-preincubated bone marrow compared with control cultures. Grade 1, adherent layer only patchy; grade 2, large adherent connected areas; grade 3, surface totally covered
Table I. Recovery of GM-CF'C after one-stage
LTBMC of VP 16-213-treated normal bone marrow (% of control)
Table 2. Recovery of GM-CFC after one-stage LTBMC of VP 16-213-treated bone marrow of patients with acute leukemia in complete remission (% of control) 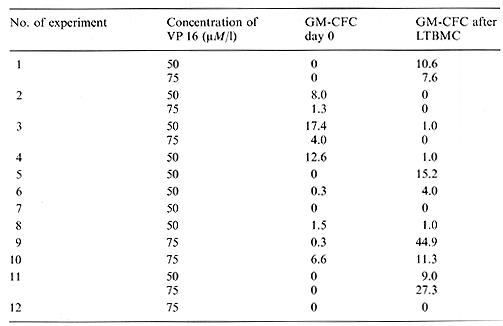 Table 3. Recovery of GM-CFC after two-stage LTBMC compared with GM-CFC after one stage L TBMC of VP 16-213-treated bone marrow of patients with acute leukemia in completeremission (% of control) 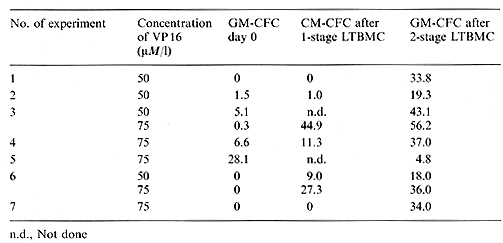
VP 16-213 is known as a cell-cycle-dependent agent affecting cells in the Sand G-2 phases [5, 6]. It shows a strong effect on GM-CFC, a population with a high number of proliferating cells. The possibly higher sensitivity of bone marrow from patients with acute leukemia in complete remission, shown by the lower than normal mean GM-CFC recovery, could be caused by a higher number of proliferating GM-CFC after chemotherapy. The higher recovery of GM-CFC after 3 weeks in one-stage LTBMC of normal bone marrow could indicate less damage to earlier stem cells, containing a lower number of cycling cells. These results agree with those of Ciobanu et al. [1] and Kushner et al. [7], who have also found a higher recovery of post-LTBMC GM-CFC after VP 16-213 incubation. However, the behavior of bone marrow of patients with acute leukemia in complete remission, the real target of purging procedures, was very inconsistent in one-stage LTBMC after treatment with VP 16-213. This may reflect different answers to the hematopoietic stress of chemotherapy, i. e., a different activation of the early stem cell pool. Otherwise, it was obvious that the establishment of the adherent layer on one stage LTBMCs was also delayed by drug treatment. Because the maintenance and survival of stem cells in LTBMC depends on an intact adherent layer, representing the hematopoietic microenvironment [8, 9] the possibility cannot be excluded that we measured a resultant of stem cell and stromal effects in our one-stage LTBMC system. To overcome this problem we used the two-stage LTBMC, where the adherent layer is preformed. The result was a much better recovery in this system, so we may assume that our first results with one-stage LTBMC indeed reflected both stem cell and stromal damage.
With a view to the establishment of purging methods it is necessary to investigate complete-remission bone marrow as the real target of purging, rather than bone marrow from healthy donors. The results of LTBMC are superior to those of GM-CFC where the hematopoietic recovery of bone marrow is concerned. One-stage LTBMC after bone marrow manipulations may reflect mixed hematopoietic/stromal effects. The use of two-stage LTBMC allows the evaluation of the stem cell recovery without the possible influence of a damaged microenvironment. Acknowledgment.
|