|
* University of Minnesota Hospitals and Clinics, 420 Delaware Street S.E., Minneapolis, Minnesota 55455, USA A.Introduction Bone marrow transplantation is now in frequent use for the treatment of a number of hematologic diseases, including severe immune deficiencies, aplastic anemias, and acute and chronic leukemias. In allogeneic transplantation, a problem remains with graft versus host disease-producing immunocompetent cells which con taminate the marrow. In autologous transplantation for treatment of the leukemias, residual leukemic cells may result in the return of unwanted cells to the recipient. Thus, an objective for both autologous and allogeneic marrow transplantation has become the removal of these unwanted cells prior to infusion into the recipient. In our institutions, the approach to the problem of purging marrow of unwanted leukemic or GVHD-producing cells has been the development of monoclonal antibodies that bind to surfaces of leukemic or GVHD-producing cells. Such monoclonal antibodies have several advantages over alternative means of purging marrow. First, an advantage over pharmacologic means is that the antibodies are highly specific for molecular determinants that are characteristic of particular cells. Second, these monoclonal antibodies have the advantage over previously produced heteroantisera in that large quantities of highly specific antibody may be produced in a very standardized manner. The current approach taken by ourselves and others is to utilize antibodies which are well characterized with respect to binding of unwanted cells, but do not bind to stem cells. Highly specific reagents can be used in this way to treat marrow in vitro without the need to administer potentially toxic substances to patients in vivo. An additional reason for the in vitro use of such reagents is to avoid the uncertain and often toxic effects of agents administered in vivo. With the use of antibodies in vitro, an adequate killing mechanism must be provided. In vivo killing mechanisms, such as through complement activation, are not sufficiently dependable to be reliable, as we observed in our earlier studies of marrow purging [I]. Thus, our recent studies have focused on in vitro killing. We have used antibody plus rabbit serum as a complement source [2], or alternatively, antibody conjugated to the potent toxin, ricin, derived from the castor bean [3]. These antibody-ricin conjugates which represent a new class of pharmacologic reagents have been developed at the National Institute of Mental Health [4, 5]. We have found both complement and ricin-mediated killing to be effective in vitro [2, 3] and studies are currently underway comparing the two forms of cell killing. Antibody-ricin immunotoxin conjugates have an advantage over antibody alone in that they can be produced in standardized form without reliance on the complex complement cascade. Not all antibodies produce effective ricin conjugates, however, in that high affinity antibodies are generally required for efficient specific killing [6]. Table I. Monoclonal antibodies
and immunotoxins for marrow transplantation; purging bone marrow
of ALL or GVHD-producing cells 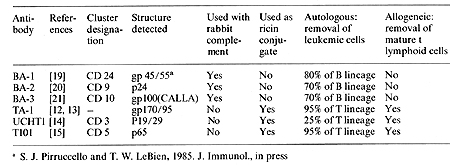
A major use for monoclonal antibodies is to purge marrow of GVHD-producing T lymphocytes. A great deal of evidence has accumulated to indicate the T Iymphocytedepleted marrow can result in effective engraftment without GVHD, despite transplantation across major transplantation barriers in the rodent [7, 8]. In our studies, marrow has been effectively depleted of T lymphocytes in mice by antibody plus complement [8] or in more recent experiments by antibody conjugated to ricin [9]. The ricin molecule is composed of two chains, A and B. The A chain is responsible for killing via inhibition of protein synthesis at the level of the 60 S ribosome. Conjugates containing B chain generally kill more effectively and exhibit more efficient cell killing per occupied receptor than conjugates made with A chain alone [ 10]. Ricin B chain binds to branched galactosyl residues on the cell surface; conjugates containing B chain are made specific for the target cell by blocking ricin binding to non-target cells with lactose [ II ]. In our clinical studies, intact ricin is currently in use. Ricin used in these experiments is conjugated using a heterobifunctional cross-linker resulting in a thioether linkage [5]. We have extensively studied three anti- T lymphocyte antibodies, each of which binds to a unique determinant on T cell surfaces {Table I). The antibody TA-l binds to a gp 170/95 kilodaltons structure as previously described [12, 13]. The antibody UCHTI is a CD3 antibody which binds to a p19/29 kilodalton structure as previously described [14]. The third antibody, T101, is a CD5 antibody, which binds to a p65 kilodalton structure as previously described [15]. These three antibodies have been conjugated to ricin as already discussed. The antibody-ricin immunotoxin conjugates have been studied extensively relative to inhibition of T cell function in the PHA assay, the generation of cytotoxic T lymphocytes, and the inhibition of stem cell growth [3]. These studies indicate that while each of these conjugates individually are effective in cellular killing, T lymphocyte activity can be further reduced by about 1 log when the conjugates are used as an equal part mixture, designated TUT. Based on preclinical studies suggesting the high efficiency of the TUT-ricin immunotoxin cocktail for T cell removal, we have proceeded to phase I-II clinical studies in which HLA-matched sibling donor marrow has been treated in vitro with the TUT-ricin cocktail prior to administration to sibling recipients [16]. Preliminary results indicate that marrow engraftment is extremely prompt, indicating no undue toxicity to marrow stem cells. No toxicity has been observed in the patients following administration of the immunotoxin-treated marrow [16]. To date, a total of eight patients have been followed for a sufficient period for evaluation (Filipovich et al., in preparation). Severe GVHD was observed in none of the patients and two developed steroid-responsive Grade 1-2 GVHD. Of concern was the fact that one patient, who showed prompt engraftment, subsequently had graft failure, presumably due to rejection. Based on these preliminary observations a phase III trial of TUT immunotoxin-treated marrow in HLAmatched combinations appears warranted. Preclinical studies have been performed using T antibodies conjugated to ricin for autologous marrow transplantation in T cell acute leukemia. These studies indicate that the antibodies TI01 and TA-l bind to most T cell leukemias and that killing in a clonogenic assay is extremely effective, particularly with TIOI, where greater than 5 log of killing was observed [ 17]. Three patients with acute T cell leukemia have had autologous marrow treated with the T cell antibody~ricin conjugates and in each case prompt engraftment was observed (Kersey et al., in preparation). The first patient treated in this manner had skin nodules prior to intensive treatment with total body irradiation and cyclophosphamide. She subsequently relapsed in the skin, suggesting, that the relapse occurred from inadequate treatment of leukemia in vivo, rather than from inadequate removal of leukemia cells from the marrow. This case illustrates that when adequate control is obtained of leukemia in vivo, efficacy of marrow cleanup will be easier to ascertain. Phase I-II studies of T cell antibody-ricin conjugates for transplantation in T leukemia continue in our institution.
The vast majority of cases of acute lymphoblastic leukemia appear to be derived from B lineage progenitor cells based on studies of immunoglobulin gene rearrangement and immunoglobulin gene expression; consistent with these observations are the data indicating that B lineage-associated antibodies bind to these leukemias [18]. Three B lineage-associated ALL antibodies have been produced and extensively studied at Minnesota. These are BA-I, BA-2, and BA-3 (Table I). BA-I binds to about 85% of cases of ALL, but does not bind to multipotent stem cells [19]. BA-2 is an antip24 antibody that binds to most B lineage ALL, but not stem cells [20]. BA-3 is an anti-gpIOO/CALLA antibody [21]. The three antibodies have been recently shown to be very effective as a cocktail with complement for the killing of ALL cells in a clonogenic assay [22]. Based on the preclinical studies describing the efficacy of that cocktail ofBA-I, 2,3 for removal of clonogenic cells in the presence of complement, we have begun phase I-II clinical trials in ALL. High risk patien ts whose leukemic cells are BA-I, 2, or 3 positive are eligible. Patients are generally those who have previously relapsed and are back in remission. Remission marrow is treated and stored while the patient recieves intensive therapy and total body irradiation and cyclophosphamide. To date, 21 patients have been treated and followed at least 2 months. Preliminary analysis indicates that patients have generally had prompt engraftment, consistent with the in vitro studies demonstrating lack of stem cell reactivity of the cocktail of BA-I, 2, 3 plus complement (Ramsay et al., 1985, Blood, in press). The only toxicity of the treatment was observed in several patients early in the study who recieved marrow that was contaminated with gram-positive organisms, presumably a consequence of marrow collection and manipulation. This is currently an ongoing study in our institution and similar to studies under way elsewhere.
Extensive preclinical studies have been performed with the use of monoclonal antibodies and antibody-ricin immunotoxin conjugates for purging marrow of unwanted cells. Purging of marrow has now been used in phase I-II clinical trials for both autologous and allogeneic marrow transplantation. The lack of in vivo toxicity of antibody or ricin and the lack of apparent stem cell toxicity is encouraging. Efficacy of marrow purging for removal of GVHDproducing cells in allogeneic transplantation or leukemic cells in autologous transplantation will be determined following additional clinical studies.
The authors thank Hybritech, Inc., San Diego, for sufficient quantities of BA-I, 2, 3, TA-l, and TI01 and Peter Beverly for UCHTI used in the studies described herein. These studies were supported in part by Hybritech, Incorporated and the following grants from the National Cancer Institute: POI-CA21737, ROI-CA-25097, and ROI-CA-31685. T. LeBien is a scholar of the Leukemia Society of America, A. Filipovich is a Clinical Investigator of the NIH.
1. Filipovich AH, Ram say NKC, Warkentin P, McGlave PB, Goldstein
G, Kersey 1H (1982) Pretreatment of donor bone marrow with monoclonal
antibody OKT3 for prevention of acute graft-versus-host disease
in allogeneic histocompatible bone marrow transplantation. Lancet
1/8284: 1266-1269 |