|
Department of Neoplastic Diseases, the Mount Sinai School of Medicine, New York, New York 10029, USA Introduction During the last ten years immunotherapy has become an important
tool in the treatment of human leukemias. Mathe et al. [1,2] demonstrated
the therapeutic value of irradiated allogeneic myeloblasts in combination
with BCG in treating childhood lymphoblastic leukemia. Similar studies
were conducted by Powles et al. [3] and Gutterman et al. [4] in
patients with acute myelocytic leukemia involving chemotherapy with
or without irradiated allogeneic myeloblasts plus BCG. These studies
consistently show that immunized patients sustain a somewhat longer
remission duration than those without immunization. Also, after
the first relapse immunized patients are reported to have higher
frequency and greater "ease" of reinduction. BCG has been used in
conjunction with cultured leukemia cells in the immunization of
patients with chronic myelocytic leukemia by Sokol et al. [5]. Under
optimal conditions prolongation of median survival of CML patients
was attained in patients who were treated with busulfan and immunotherapy
as compared to controls who received busulfan alone. In an attempt
to find a more standardized immunological adjuvant, Weiss et al.
[6] conducted extensive studies with MER, the methanol extraction
residue of BCG. They were able to demonstrate therapeutic advantage
of MER, especially in murine leukemias.
The chemotherapy protocol on this study is based in maximal chemotherapeutic reduction of leukemic burden. This is achieved by induction therapy with a regimen of cytosine arabinoside continuously administered in travenously for 7 days at lOO mg per square meter of body surface area per day, and daunorubicin at a dose of 45 mg per square meter ofbody surface area per day by direct injection on days I. 2 and 3. This regimen has induced approximately 70 per cent of patients into remission. All patients were between the ages of 15 and 70. All received cyclical maintenance chemotherapy every 4 weeks. This consisted of 5 day courses of AraC in addition to 6-thioguanine. cyclophosphamide, CCNU, or daunorubicin sequentially with each course repeated at 4 months cycles.
Patients became eligible for collection of myeloblasts after satisfying
the following criteria: Negative HA-A as determined by radioimmunoassay,
no previous chemotherapy. total WBC higher than 25000/µ1, and higher
than 70% myeloblasts in the peripheral blood. The myeloblasts were
obtained by leukophoresis. In the last five years we collected myeloblasts
from 93 patients between the ages of 14 and 72 years and have not
encountered any important side-effects during the two to four hour
procedure. After leukophoresis. the myeloblasts were separated from
contaminating red blood cells by sedimentation at 37°C. After sedimentation
leukemic cells were mixed -with special freezing media (free of
calcium and magnesium) containing 15% autologous or AB plasma and
10% DMSO. The final cell concentration was 0.3 -1,0 X 10 high 8
cells/mI. Myeloblasts were frozen by programmed freezing at a temperature
drop of 1,5°C per minute until -38°C was reached. and then rapidly
to 80°C. The frozen cells were immediately stored in the vapor phase
of Treatment of Allogeneic Myeloblasts with Neuraminidase Myeloblasts were thawed and were washed twice with mixed salt and glucose media at 4°C and further purified on a 22 per cent human albumin gradient. layered over 45% surcrose for the separation of viable from nonviable blast cells. After purification, blast cells were washed and incubated with N'ase at a concentration of 50 units of enzyme per 5 X 10 high 7 cells/mi in sodium acetate buffer, for 45 minutes at 37°C. The cells were then washed and resuspended in physiological saline and used as immunogen within 30 minutes.
Immunization with neuraminidase treated allogeneic myeloblasts was performed by intradermal injections. In order to get maximum exposure to the immunogen, sites were widely spread in the supraclavicular, infraclavicular, arm. forearm. parasternal, thoracic, suprainguinal and femoral regions draining into several node bearing areas. Dose dependent cellular titration was performed with each immunization with 0,5, 1,5, 2,5 X 10 high8 and 0 cells. The total immunization load was about 10 high 10 cells at 48 body sites. The injections of neuraminidase treated myeloblasts produced no local lesions other than the delayed type cutaneous hypersensitivity reaction (Table, I) and none of the patients developed chill, fever. or adenopathy. No hypersensitivity reaction was apparent at the site of injection of physiological saline, heat denatured neuraminidase. or supernatant of cell incubation media. In patients randomized to receive MER too, we used ten intradermal sites of lOO µg/.l ml each totaling 1.0 mg of MER. Table 1. Delayed hypersensitivity response
to X-irradiated or neuraminidase treated myeloblasts 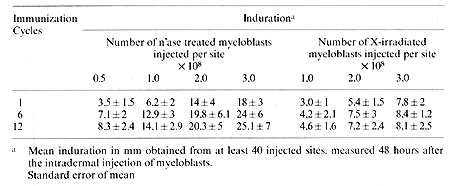
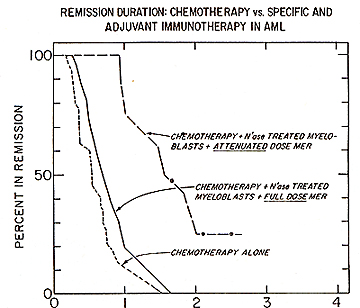
Based on experimental observations, a successful chemoimmunotherapy trial was conducted in patients with acute myelocytic leukemia. The interim analysis presented below is calculated by standard life table methods and is subdivided into several subsets. The data represents 91 patients with AML who were allocated in three groups following successful remission induction using cytosine arabinoside and daunorubicin. Patients designated to receive immunotherapy were injected (i.d.) in approximately 48 sites every 28 days with 10 high 10 N'ase treated allogeneic myeloblasts. For 27 of the 91 acute myelocytic leukemic patient. the remission duration on the chemotherapy alone was 243 days: for those receiving N'ase modified allogeneic myeloblasts as immunogen the mean remission was 686 days (Fig. 1 ). The difference in remission duration between the two treatment groups is highly significant: p = .00 1 using Breslow's. Logrank and Cox regression analysis. Combination of specific plus adjuvant immunotherapy did not act synergenistically in the treatment of AML patients. Fig.2 shows the behavior of patients immunized with N'ase treated myeloblasts plus the full prescribed dose of MER with a mean remission duration of 336 days. This was compared to another group of patients. in whom. based on the demonstration of the presence of suppressor cells and supporting clinical evidence, the MER dose was attenuated or omitted. This modality provided considerable improvement in the remission duration (of 630 days) but still not has reached the level attained with N'ase treated myelobasts alone (see Fig. 1). However the difference between the control vs. cell + MER is significant at p = .03. It appears that inclusion of MER in this immunotherapy protocol adds no value to chemoiillillunotherapy when added to N'ase treated allogeneic myeloblasts as immunogen in AML patients.
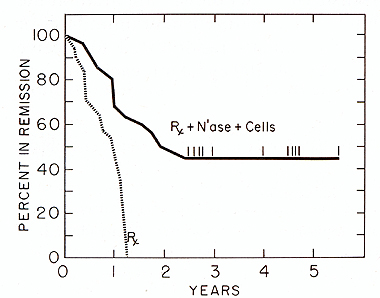 Fig.1. Duration of complete remission in acute myelocytic leukemia in patients immunized with neuraminidase treated allogeneic mveloblasts.
REMISSION IN YEARS AFTER M1
Fig.2. Effect of chemotherapy and chemotherapy plus neuraminidase treated allogeneic myeloblasts plus MER on remission duration in patients with AML
The in vivo immunological status of the immunotherapy patients
at various stages of their treatment was measured by DCH response
to five recall antigens: PPD. mumps, candida, varidase and dermatophytin.
Interpretation of the skin tests was based on the induration as
measured in millimeters in two directions at 48 hours and considered
positive if the diameter of induration exceeded 5 mill. Fig. 3 shows
that there were significant improvements in the response to recall
antigens in patients immunized with N'ase treated myeloblasts. However,
patients who received N'ase treated myeloblasts plus MER. after
an initial improvement. the DCH response to recall antigens gradually
declined and was ultimately eradicated. The decline of in vivo response
to antigens often preceeded subsequent relapse of those individuals
who received full dose of MER in addition to N'ase treated myeloblasts.
Impact of Immunotherapy on the Immunological Status of AML Patients
On each immunization day, remission lymphocytes were isolated from
freshly drawn heraranized blood by the Ficoll-Hypaque gradient method
for the following in vitro assays: Surface markers by E and EAC
rosettes, 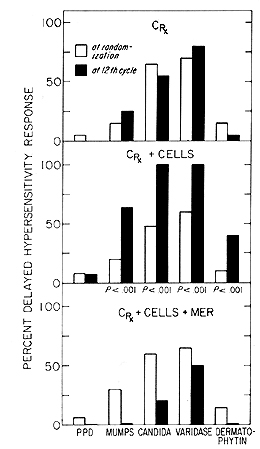
phytohemagglutinin (PHA) and pokeweed mitogen (PWM) induced Iymphoblastogenesis
and tumor leukocyte culture (MLTC) with immunizing allogeneic myeloblasts.
Although the quantification of E and EAC rosette forming lymphocytes
from AML patients in the protocols were routinely performed, we
are only showing in Table 2 two test periods: a) The initial E and
EAC values at the time of randomization, and b) the impact of immunotherapy
on the T and B lymphocyte surface markers. The median value for
normal donors of Erosetting PBL is 74,4%, with 1,986 as the number
of absolute T-lymphocytes. For EAC rosetting the normal PBL values
are 22,1% with 521 as the number ofabsolute lymphocytes. Patients
at the time of randomization, still under recovery from induction
and consolidating chemotherapy, have shown significantly lower percentage
(49,2 and 51,7) and absolute number ( 412 and 487) of T -cells as
well as lower percentage ( 16,2 and 16,5) and absolute number (169
and 195) of EAC rosetting lymphocytes. Patients in both chemotherapy
regimen showed a significant increase of T- and B-lymphocytes as
compared to values at the time of randomization both in percentage
and in absolute number. Maximum lymphocyte blastogenesis was attained
at 0,15 µg per well for PHA and 30 µg per well for PWM, for normal
donors, as well as for the remission lymphocytes from patients in
either of the therapeutic regimen. Lymphocytes obtained from patients
receiving chemotherapy alone showed consistently lower degree of
stimulation to both mitogens all through the observation periods.
Lymphocytes obtained from AML patients who have been immunized with
N'ase treated myeloblasts showed, despite the tact that they have
been receiving chemotherapy, nearly normal lymphocyte function (Table
2). Countraiwise, patients immunized with N'ase treated myeloblasts
plus MER have shown in the first six months of immunotherapy a continuous
improvement in their response to mitogens but not to tumor cells.
This was followed by a gradual decline in lymphocyte function. The
fact that patients treated with N'ase treated myeloblasts plus MER
have similar E-rosetting PBL as patients treated with cells alone,
but have significantly altered in vivo and in vitro lymphocyte function
(Fig. 4), raised the possibility of the presence of an inhibitory
mononuclear cell popula tion in the blood of such immunized patients.
This hypothesis was tested and the data are summarized in Fig. 5.
Isolated enriched T-cell fractions trom normal donors or from patients
immunized with N'ase modified myeloblasts gave similar uptake of
H3TdR as the unseparated PBL. However, isolated enriched T -cell
fraction from AML patients who received N'ase treated myeloblasts
plus MER, and have shown declining in vivo and in vitro immunological
responses, gave 3-7 times greater H3TdR incorporation in response
to PHA than their unseparated PEL. The response otthe enriched T
-cells was strongly inhibited by addition of autologous but not
normal donors' adherent mononuclear cells. These findings suggest
that depression of cell mediated immunity is seen in most of the
tested AML patients who received N'ase treated myeloblasts plus
full dose of MER, but not among the patients immunized with N'ase
modified myeloblasts alone, maybe due to the suppression of certain
T -cell functions by circulating monocytes affected by MER. The
time of appearance of the apparent suppressor cell activity was
different from patient to patient and omission of MER from the treatment
in most cases prompted recovery of the patients' in vivo and in
vitro immunological parameters and a gradual decrease of suppressor
cell population. 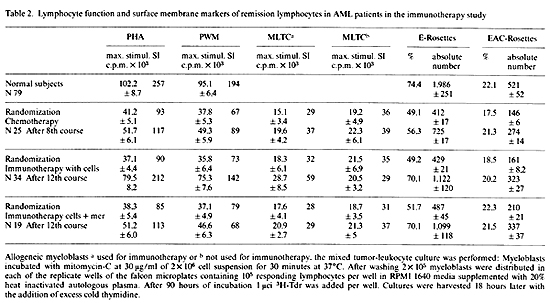 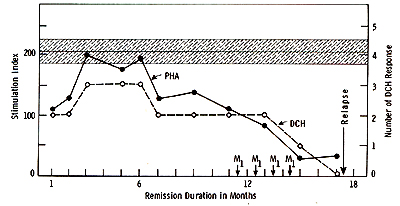 Fig.5. Appearances of suppressor cell activitv in AML patients treated with N'ase treated myeloblasts plus MER
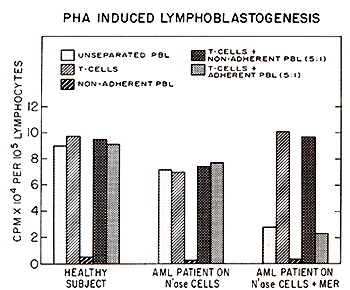 Fig.4. Impact of N'ase modified myeloblasts plus MER on various immunological parameters in AML patients
Our studies clearly show that significantly longer remission duration was attained in groups ofAML patients immunized with neuraminidase treated allogeneic myeloblasts as compared to patients who received chemotherapy alone or neuraminidase treated myeloblasts plus MER. It is clear that MER. albeit apparently active alone in certain other clinical studies impairs the immunotherapeutic value of neuraminidase treated allogeneic myelo blasts in AML patients. The in vivo and in vitro immunological test results reflect the host's immunological status in each arm of the protocol and correlate well with the duration of remission achieved with specific vs. combination of specific plus adjuvant immunotherapy.
This work was supported in part by grant and contracts CA-I-5936-02. CA-5834. NCI Cancer Virus Program No. I-CB-43879. and NCI Immunotherapy Program No. I-CO-43225 and The T.J. Martell Memorial Fund. We express our appreciation to the Behring Institute. Behringwerkc AG. Marburg. Federal Republic of Germany. for supplying the highly purified neuraminidase for this study. We also thank Suzan Sattler-Gillman. RN. and Angeles Mison for the excellent assistance in performing the immunotherapeutic manipulations and Rogena Brown for secretarial assistance.
I. Mathe. G.. Pouillart. P.. Lapeyrague. F: Br. J. Cancer 23,
814-824 (1969) |