|
Cellular und Tumor Immunology Section Laboratory of Cell Biology National Cancer Institute Bethesda, Maryland 20014 USA Cellular immune reactions are generally thought to playa major role in host resistance against tumor growth. Human acute leukemia cells have been found to contain tumor associated antigens, and it is possible to measure the cell-mediated immune response to these antigens. In addition to specific reactivity, it is quite important to evaluate the functional integrity of the cellular immune system in leukemia patients. The disease process itself or the chemotherapeutic agents could cause general depression in reactivity. The techniques currently being used for such studies are summarized in Table 1. In this paper, we will review the information available from each of these approaches, and discuss their possible relevance to the clinical state . Table 1. Assays of Cellular Immunity in
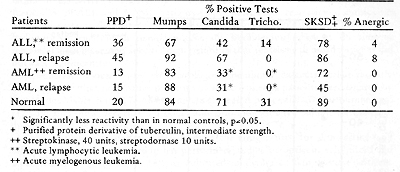
Human Acute Leukemia 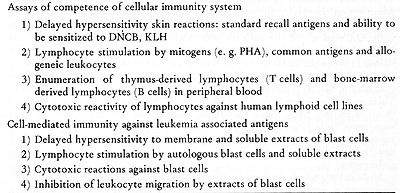
Three aspects of the competence of the cellular immunity system in acute leukemia patients are of interest: a) the effect of the tumor burden on reactivity; b ) the effect of chemotherapy; and c) the inherent ability of patients to have cellmediated immunological reactions against a variety of antigens. Unfortunately, it is difficult to clearly study each of these factors. The ideal time to obtain information on the patient's inherent reactivity would be prior to development of disease. Based on the theory of immunological surveillance ( 1, 2 ), one might anticipate that individuals developing leukemia have depressed immunological competence. However , it is not possible to obtain information at this time. It is practical to examine patients at the time of diagnosis, when tumor is present, and during clinical remission. Since chemotherapeutic agents are used to induce remission, their effects must also be considered. Skin tests for delayed hypersensitivity have been extensively used to study cellular immunity in acute leukemia patients. In studies before thereapy ( 3) and shortly after the onset of therapy ( 4) , decreased reactivity of some patients to standard recall skin test antigens was found. Hersh et al ( 4) found that depressed skin reactivity was associated with poor response to therapy for six months or longer had even less reactivity than that seen in initial studies ( 5) .Chemotherapy may have had an important influence on these results. Borella and Webster (6), in a study of children with acute leukemia in remission, observed that long-term combination chemotherapy had immunosuppressive effects on skin test reactivity. Some treatment protocols appeared to be more immunosuppressive than others. Many of the patients of Hersh et al ( 4, 5) received COAP, which was noted to be very .. ImmunosuppressIve. Our laboratory also studied the response of ALL (acute lymphocytic leukemia) and AML (acute myelogenous leukemia) patients to a battery of standard recall antigens (7). We performed almost all of our tests after induction of remission, or at the time of bone marrow relapse. Patients at the National Cancer Institute are usually treated with combination hemotherapy, with repeated cycles of one week of treatment followed by two-three weeks off. To reduce the possible effects of treatment on the results, the patients were usually skin tested just prior to a course of therapy. Table 2 gives a summary of our data. Ninety-six percent of patients with ALL and all of the patients with AML reacted with at least one of the antigens, when tested in remission or in relapse. There were no significant correlations between the incidence of reactivity to anyone of the particular antigens and clinical state, time of test in relation to chemotherapy, or length of survival. The reactivity of the ALL patients to PPD was high, due to immunotherapy with BCG. There are several possible explanations for the differences between our results and those of Hersh's group. Since they found that patients with intact skin reactivity were more likely to go into remission ( 4) , it is possible that our patients, initially tested in remission, were a selected population. The type of chemotherapy used, and the timing of tests in relation to therapy, may also have been important differences. Stimulation of patients' lymphocytes by mitogens, recall antigens and allogeneic leukocytes is another widely used technique for assessing immune competence. Table 2. Skin Reactions to Recall Antigens
 Hersh et al ( 4) found that one-third of leukemia patients studied had decreased responses to phytohemagglutinin and streptolysin 0. As with the skin tests, most of these poorly reactive patients did not respond well to therapy. I t has been found the time of testing, in relation to chemotherapy, has an important influence on the results ( 8, 9, 10) .Reactivity was greatest 1 o~ 20 days after cessation of therapy, and in fact was sometimes higher than normal reactivity at this time. Techniques have recently been developed which may allow enumeration of thymus-derived lymphocytes (T cells) and bone marrow-derived lymphocytes (B cells) in the peripheral blood. T cells ,appear to have receptors for sheep erythrocytes ( E ) , while B cells have receptors for the third com ponent of complement and can thereby bind E coated with antibody and complement (EAC). Rosette assays based on binding of E and EAC are easy to perform and may provide useful information in cancer patients. Many cancer patients have decreased percentages of circulating T cells (11,12). Sen and Borella (13) have found that longterm chemotherapy caused depression in lymphocyte counts, and B cells were decreased more than T cells. After cessation of therapy, the B cells returned to normal levels within two to three months, whereas recovery of T cells took longer . No systematic serial study of these cell populations at different phases of disease have been reported as yet. Rosenberg et al ( 14) have recently found that the lymphocytes of most normal individuals have cytotoxic reactivity against human lymphoid cell lines. McCoy et al ( 15) found that many patients with solid tumors and patients with immune deficiency diseases had significantly reduced activity. Fig. 1 shows the results with leukemia and lymphoma. Many of the leukemia patients had low reactivity. Reactivity was found to vary at different times in relation to chemotherapy (16). However, no consistent pattern was seen among different patients. Patients who were off therapy for four to eight weeks (points labelled Rx in Fig. 1) had normal or high reactivity. 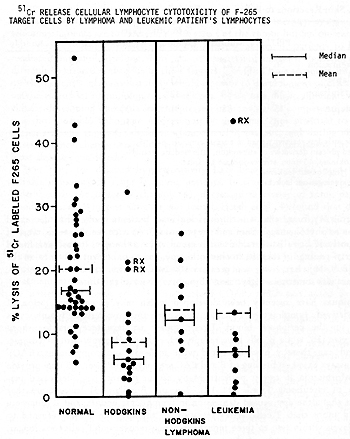
Cell-Mediated Immunity Against Leukemia Associated Antigens Skin testing with membrane or soluble extracts of blast cells has
been used to measure delayed hypersensitivity reactions to leukemia
associated antigens (7, 17) . Table 3 is a summary of our tests
with autologous membrane extracts of blast and remission cells (7).
In patients with ALL who were in remission, positive reactions to
autologous blast extracts were obtained in 20 of 44 tests. ALL patients
tested in relapse gave only one positive reaction. In AML, there
was also a significant correlation of reactivity to blast extracts
and clinical state. Serial skin tests were performed in 29 ALL and
8 AML patients (7 ). In most cases, reactivity to autologous blast
cell extracts fluctuated in parallel with changes in clinical state.
Patients who had positive reactions in remission generally became
unreactive at the time of relapse. This represented specific depression,
in that reactions to recall antigens did not vary significantly.
In contrast to our results with autologous membrane extracts, it
has recently been reported that no positive reactions of acute leukemia
patients were elicited by autologous viable cells ( 17) or mitomycin-C
treated blast cells ( 3) .Gutterman et al ( 17) did find, however,
that 3M potassium chloride extracted soluble antigen produced positive
skin reactions. These studies indicated that the form of the antigen
used for skin testing may influence the results. Our group has performed
studies on antigens solubilized and separated from AML cells (18),
as indicated in Figure 2. Skin reactive antigens could be solubilized
by low frequency sonication, and then separated by Sephadex G-200
and DEAE-cellulose chromatography. Table 4 gives the representative
results of testing a patient with autologous DEAE fractions. Skin
reactions were elicited by two of the fractions from blast cells,
and not by the comparable fractions from remission cells. I t has
been possible to further separate the AML skin reactive antigens,
by gradient acrylamide gel electrophoresis. Tabel 5 shows a test,
in which only one region of the gel containing the blast extract
gave a positive reaction. No reactions were elicited by the comparable
remission cell fractions. 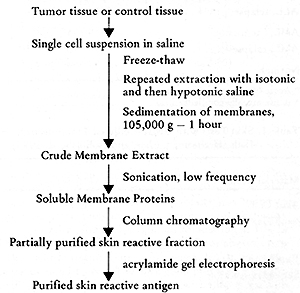
Table 3. Skin reactions to Autologous
Membrane Extracts 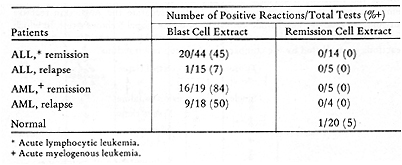 Table 4. Skin Tests with DEAE-Cellulose Fractions of Soluble Autologous AML * Cells (Herberman, Char, and Hollinshead, 1973) 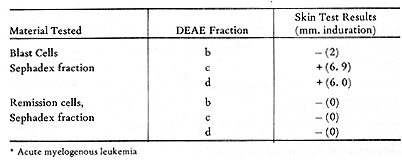 Table 5. Skin Tests with Acrylamide Gel Fractions (Herberman, Char , and Hollinshead, 1973) 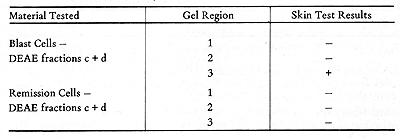 Table 6. Skin Reactions to Allogeneic Membrane Extracts 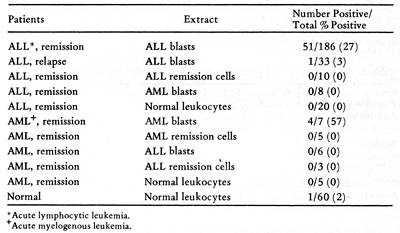
Table 7. Skin Reactions to Extracts of
Lymphoid Cell Lines 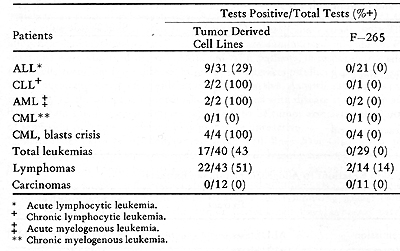

Table 9. Cell-Mediated 51 Cr Release Assay
-Allogeneic Target Cells 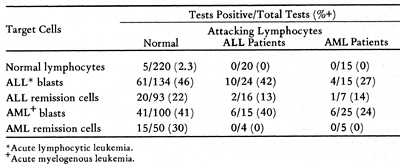 Table 10. Results of three assays of cellular immune reactivity in acute leukemia to autochthonous blasts cells 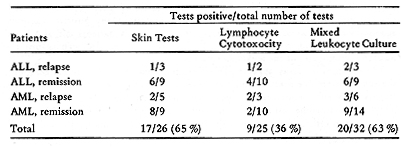
There has been much recent interest in the use of immunotherapy in acute leukemia. Most of the studies already performed have been empirical, without assessment of the antigenicity of the immunizing cells and without immunological monitoring of the immune response to the therapy. An immunotherapy study was performed on previously treated ALL patients, in which allogeneic ALL blast cells plus either BCG or methotrexate were given ( 24) . Immune responses were serially determined, by skin testing and by lymphocyte stimulation. The most dramatic change was in the response of the BCG treated group to PPD. Skin tests with allogeneic blast extracts did not provide clear evidence for immunization, even against the HL-A antigens of the donor cells. There was, however, a correlation between skin reactivity against the extract of donor cells and the duration of remission. In the lymphocyte stimulation assays, there was also little evidence for immunization by the donor cells. The use of these immunological assays could help in designing future immunotherapy trials. Allogeneic cells could be selected on the basis of their ability to elicit skin reactions, thereby documenting that the donor cells possess common antigens. In addition, monitoring with skin tests could be used to determine the immunogenicity of different immunizing preparations and schedules. It would be very helpful if autologous cells were available for skin testing and for in vitro tests, since they would permit a distinction between immunization to leukemia associated antigens and immunization to normal histocompatibility antigens. If an immunotherapy trial were clinically successful, it would provide important information on the predictive value of each of the assays.
1. Burnet, F. M.; Brit. Med.J. 1: 779 and 841,1957. |