Infections during granulocytopenia are
a major complication of autologous bone marrow transplantation [2,
5]. Since recombinant human granulocytemacrophage colony-stimulating
factor (rhu-GM-CSF) has proved to accelerate bone marrow recovery
after cytostatic chemotherapy [1] and autologous bone marrow transplantation
(ABMT) [3, 4], we studied its effects on hematopoietic regeneration
and on infectious complications in a controlled trial.
This was a prospective placebo-controlled double-blind study in 21
centers for BMT in Europe and Israel. A total of 81 patients with
acute lymphoblastic leukemia in complete remission or with non-Hodgkin's
lymphoma were treated after total-body irradiation of at least 8 Gy
followed by hig-dose chemotherapy. They received either 250 µg rhu-GM
-CSF/m² ( Escherichia coli derived) daily by continous infusion after
ABMTor placebo. Treatment was continued until the neutrophil count
reached >500 µ1 for 1 week. The maximum treatment duration, however,
was 30 days. The data on 39 patients in the rhu-GM-CSF group and on
40 in the placebo group were evaluable. The median time needed to
reach 500 neutrophils/µ1 was 15 days with rhu-GM-CSF and 28 days with
the placebo (p=0.001; Fig. l).Bacterial infections occurred in 14
(35.9 %) of patients treated
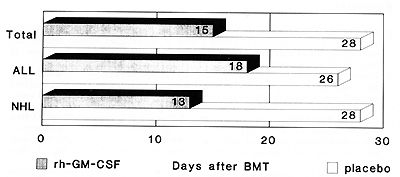
Fig.1. Duration of neutropenia after AMBT in patients
with and without rhuGM-CSF
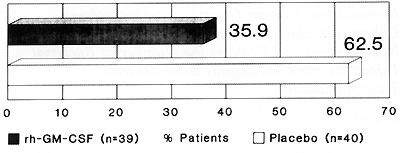
Fig.2. Number of patients with infections and bacterial
infections after ABMT with and without rhuGM-CSF. p = 0.024
with rhuGM-CSF and in 25 (62.5 %) of patients given the placebo
(p=0.024; Fig. 2). Nine of the 14 bacterial infections in the rhuGM-CSFgroup
and 20 of the 25 infections in the placebo group were diagnosed
within the first 10 days after BMT. The patients were isolated for
26 days (median duration) with rhuGM-CSF and for 30 days with placebo.
The side effects of rhuGM-CSF were reversible capillary leakage
and a higer rate of reversible fluid retention in five patients.
Patients with rhuGM-CSF had lower serum protein and albumin levels
than the palcebo group. We conclude that continous infusion of rhuGM-CSFafter
autologous BMT accelerates the regeneration of granulocytes and
reduces the number of bacterial infections significantly. RhuGM-CSF
makes it possible to shorten the duration of patient isolation and
has only moderate and reversible acute side effects. RhuGM-CSF (E.
coli) may be useful in reducing the immediate infectious complications
of this aggressive high-dose antitumor therapy and become an important
supportive therapy.
References
1. Antman KS, Griffin JD, Elias A, Socinski MA, Ryan L, Cannistra
SA, Oette D, Whitley M, Frei E III, Schnipper LE (1988) Effect of
recombinant human cranulocytemacrophage colony-stimulating factor
on chemotherapy-induced myelosuppression. N Engl J Med 319:593-598
2. Bearman SI, Appelbaum FR, Back A, Petersen FB, Buckner CD, Sullivan
KM, Schoch HG, Fisher LD, Thomas ED (1989) Regimen-related toxicity
and early post transplant survival in patients undergoing marrow
transplantation for lymphoma. J Clin Concol 7:1288-1294
3. Brandt SJ, Peters WP, Atwater SK, Kurtzberg J, Borowitz MJ, Jones
RB, Shpall El, Bast RC Jr, Gilbert Cl, Oette DH (1988) Effect of
recombinant human granulocytemacrophage colony-stimulating factor
on hematopoietic reconstitution after high-dose chemotherapy and
autologous bone marrow transplantation. N Engl J Med 318:869-867
4. Link H (1990) Recombinant human granulocyte-macrophage colony-stimulating
factor (rh-GM-CSF) for hematological reconstitution after autologous
bone marrow transplan tation. In: Mertelsmann R, Herrmann F (eds)
Hematological growth factors in clinical applications. Dekker, New
York, 313
5. Nemunaitis J, Rabinowe SN, Singer JW, Bierman PJ, Vose JM, Freedman
AS, Onetto N, Gillis S, Oette D, Gold M, Buckner D, Hansen JA, Ritz
J, Appelbau FR, Armitage J, Nadler LM (1991) Recombinant granulocyte
macrophage colony-stimulating factor after autologous bone marrow
transplantation for lymphoid cancer. N Engl J Med 324:1773-1778
| 
