Hämatol. Bluttransf. Vol 35 |
|
1 Petrov Research Institute of Oncology, Leningrad,
USSR. Introduction Multiple myeloma (MM) is a malignant clonal B-lymphoproliferative disease. Survival ranges from a few months to many years [7]. The determination of prognosis for the disease S course IS Important in therapeutic choice. At least two factors determine the course of MM: 1) the biology of myeloma cells and 2) the interrelation of malignant cells and host organism. That is why the morphology of myeloma cells is one of the most important prognostic factors of the disease [4]. There are also other prognostic factors associated with both malignant cell characteristics and the status of the host's immunocompetent system [7]. Taking into account the data on the decrease in colony-forming units -granulocyte/macrophage (CFU-GM) in MM [2, 11], and the antitumor effect of tumor necrosis factor alpha (TNFalfa) [8, 9] and interleukin-2 (IL-2) [10], we investigated myeloma cell morphology, the level of serum ß2-microglobulin (Sß2-M) and CFU-GM, TNF production by peripheral blood monocytes, and IL-2 production by peripheral blood mononuclear cells in different types ofMM course in order to determine their prognostic significance.
We investigated 101 patients with MM. Diagnosis was made according to previously described criteria [5].
It is impossible to predict individual prognosis in MM according
to the stage of the disease [6] at the first presentation. That
is why we classified our patients according to their life duration,
which determined the type of the disease. This classification is
a result of prospective investigation. We divided the patients into
three groups: first indolent myeloma, second -active myeloma, third
-aggressive myeloma. The diagnosis was made on the following criteria:
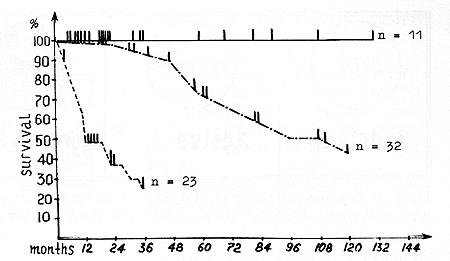 Fig.l. Survival of patients with various courses of multiple myeloma. The solid line shows the indolent course; the dash-dot line, the active course, the line of dashes, the aggressive course
The degree of myeloma cell maturation was determined according
to the following criteria of the morphological classification system
for MM [4]: 1) mature -comprising more than 50 % mature plasma cells;
Serum ß2-Microglobulin. Colony-Forming Unit-GranulocyteIMacrophage. Biological Activity of IL-2. TNF alfa Activity.
It was revealed that mature plasma cells were the morphological
substrate in indolent myeloma (Fig. 2) at the same time in active
myeloma, mature cells predominated in 77% of the patients, whereby
in the remaining 23% of the patients, the cells were immature. In
aggressive myeloma, immature cells were the main morphological type
in 56.3% of the patients, plasmablasts prevailed in 37.5 % of the
patients, and mature cells were in 6.2% of the patients. We discovered
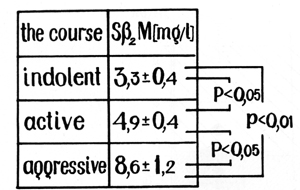
that the level of Sß2-M correlated with the course ofMM in most
of the patients (Fig. 3). There was a significant difference between
the level of Sß2-M in patients with indolent myeloma (3.3 +- 0.4
mg/l) and in healthy controls (1.7 +- 0.4 mg/l; p < 0.05). We revealed
a significant difference between the level of 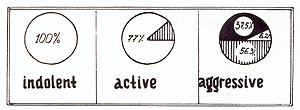 Fig. 2. Morphology and course of MM. The white area shows the percentage of mature cells; the hatched area, the immature cells; and the black area, the plasmoblastic cells
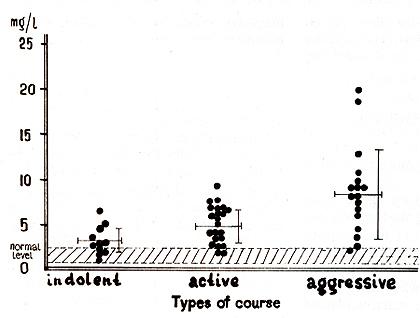 Fig. 3. Serum ß2-microglobulin and types of courses in patients with multiple myeloma
trols (13.9 +-1.2 units of activity; p < 0.05) and patients with
indolent (12.9+- 1.1 units of activity) (Figs.6, 7) and active courses
(11.9+- 0.7 units of activity) and between the indolent and active
courses. Then significant differences were found in the IL-2 production
between patients with active and aggressive myeloma (8.6+- 0.6 units
of activity; p < 0.01) and also between aggressive and indolent
myeloma and healthy controls (p < 0.01). An insignificant difference
between the pyrogen-stimulated production of TNF alfa in patients
with an indolent course (1.5+- 0.3 ng/ml) and healthy controls (2.2
+- 0.38 ng/ml) was detected (p > 0.05; Fig. 8). A significant difference
was found between the pyrogen-stimulated production of TNF alfa
in patients with indolent and active courses (6.1 +- 1.1 ng/ml;
p < 0.01 ). There was also a significant different in TNF alfa production
between patients with active myeloma and healthy controls (p < 0.05),
and also in TNFalfa production in patients with active and aggressive
myeloma (2.0 +- 1.2 ng/ml; p < 0.05). There was an insignificant
difference between TNF alfa production in patients with aggressive
and indolent courses and healthy controls (p > 0.05; Fig. 9). 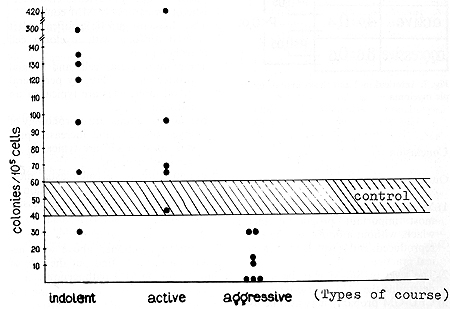 Fig. 5. Colony-forming unit granulocyte and macrophage
of bone marrow and the multiple myeloma course
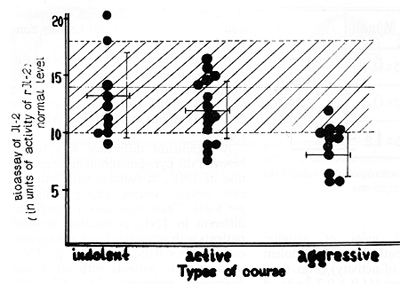 Fig.6. Production of interleukin-2 by peripheral blood
mononuclear cells in patients with different types of courses in
multiple myeloma
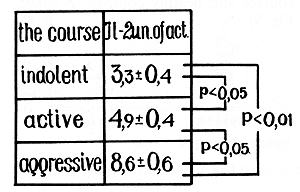 Fig.7. Interleukin-2 and the course of multiple myeloma
Our data suggest that: 1) Morphology of myeloma cells is the most
important prognostic factor which, without a doubt, can be easily
reproduced, and is widely used in clinical practice. 2) The level
of Sß2-M may be used to determine the MM course type. 3) CFU-GM
probably may also be used as a prognostic factor. An increased or
normal level of CFU-GM confirms the indolent or active course of
MM, while a decreased level of CFU-GM reflects the aggression of
MM. 4) Decreased production of IL-2 by peripheral blood monocytes
is characteristic of an aggressive MM course. 5) TNF alfa has a
prognostic significance if it is used together with myeloma cell
morphology as a) mature myeloma cells and normal production of TNF
alfa by peripheral blood monocytes are typical of an indolent course;
b) mature or immature morphology of myeloma cells and increased
production of TNF alfa are typical of an active course; and c) immature
or plasmablastic morphology and normal production of TNF alfa are
typical of an aggressive course. The studies mentioned above were
necessary in order to find out the role of these factors in MM pathogenesis.
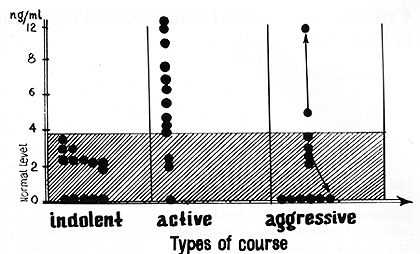 Fig.8. Pyrogenal-stimulated tumor necrosis factor-alpha
production by peripheral blood monocytes in patients with multiple
myeloma
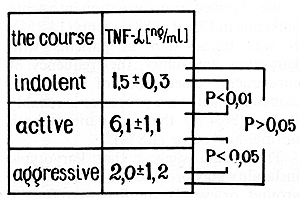 Fig. 9. Tumor necrosis factor-alpha and the course of multiple myeloma
References 1. Afanasiev BV, Tiranova SA, Kulibaba Ta, Zubarovskaya LS, Bolshakova
aD, Zabelina TS (1983) Cloning of human |