|
1 National Institute of Hematology and Blood Transfusion, Budapest,
Hungary. Introduction Based on the results of the immunophenotypical analysis both B-cell chronic lymphocytic leukemia (B-CLL) and Bcell hairy cell leukemia (B-HCL) appear to be derived from activated lymphocytes [1-4]. However, CLL-B cells represent an earlier stage of differentiation than the cells of HCL. The data of the gene rearrangement studies also suggest that HCL derives from the clonal expansion of a cell at a later developmental stage than the CLL [5]. In this study the peripheral blood mononuclear (PBM) cells of patients with CLL and HCL were characterized by the presence of a variety of cell surface dif ferentiation and activation antigens.
Twenty CLL patients were studied. Their age ranged from 42 to 79 years (mean: 63.0). The male to female ratio was 0.67: 1. Five HCL patients in the leukemic phase of the disease were selected from the 24 investigated HCL patients (age 22-79 years, mean: 54.3). All patients were untreated for at least 3 months at the time of the study. Eight healthy age- and sex-matched persons served as controls. Indirect immuno fluorescence was used to detect the dif ferentiation and activation antigens on PBM cells. T cell-associated antibodies were CD 3 (T3), CD2 (T11), CD8 (T8), CD4 (T4) from Ortho Diagnostic System and (Raritan, New Jersey, USA), CD5 (Leu1) from Becton- Dickinson (Mountain View, California, USA). B cell-associated antibodies were CD 19 (B4) and CD20 (B 1) from Coulter Corporation (Haleah, Florida, USA). Class II antigen was detected with HLA-DR from BectonDickinson. Anti-immunoglobulin M (IgM) antibody (Heintel Vienna, Austria) was used as directly conjugated with fluorescein isothiocyanate (FITC). RAB-1 monoclonal antibodies (moAbs) were used to detect the hairy cells [ 6] .The Immune Monitoring Kit of BectonDickinson was used to detect activated T cells. The monoclonal antibodies (moAbs) from the panel of the IVth International Workshop on Human Differentiation Antigens (CD 25, CD 30, CD40, CD69, CD70, CD39, CD71) were used to detect the activation antigens. Analysis of all samples was done using a F ACST AR flow cytometer (Becton- Dickinson ) .
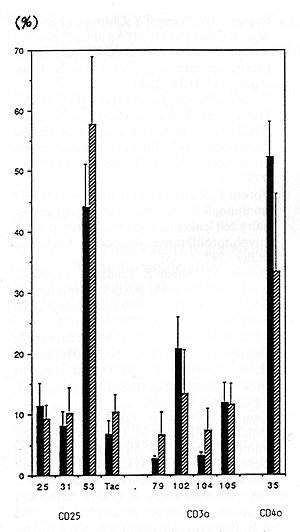
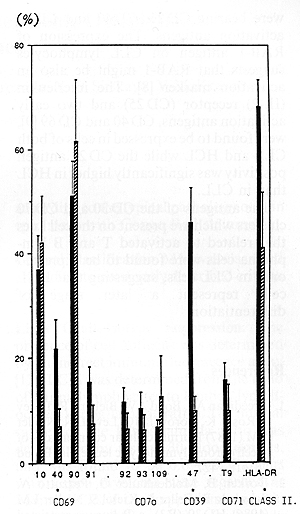
The cells of 20 CLL patients were found to be B-cell phenotype
when studied with Abs directed against CD 19, CD20, HLA-DR and surface
immunoglobulin (sIg) antigens. Furthermore, significant percentage
of the cells gave a positive reactions with moAbs to CD 5 [7]. The
PBM cells from 24 HCL patients showed similar antigen profile: CD 19, CD20, HLA-DR and sIg positivity, but CD 5 negativity. The moAbs to hairy cells RAB-1 showed a positive reaction both on CLL and HCL cells, but the expression of RAB-1 was found to be significantly higher in HCL than in CLL. Neither CLL nor the HCL cells expressed the CD21 antigen (C3d receptor, EBV receptor). Investigating the presence of various activation antigens using 18 moAbs grouped into seven clusters, both CLL and HCL PBM cells were found to carry a significant proportion of activated cells when compared to the healthy controls. Comparing the CLL and HCL cells with each other, the activation stage of PBM cells in CLL and HCL differed only in two out of the 18 activation antigens (Figs. 1, 2). The expression of the CD 39 cluster as well as the expression of a single antigen of the CD 69 clusters were significantly higher in CLL than in HCL.
Our data confirm and extent earlier suggestions that both CLL and HCL might be derived from subpopulations of activated lymphocytes. The CLL cells were characterized by the expression of CD 25, CD 30, CD 39, CD 40, CD 69 and CD 70 activation antigens, while the HCL cells were bearing CD 25, CD 40 and CD 69 activation antigens. The expression of RAB-l antigen on CLL lymphocytes suggests that RAB-l might be also an activation marker [8]. The interleukin (IL-2) receptor (CD 25) and two early activation antigens, CD 40 and CD 69 [9], were found to be expressed in cells of both CLL and HCL while the CD 25 antigen positivity was significantly higher in HCL than in CLL. The antigens of the CD 30 and CD 70 clusters which are present on the cell lines that related to activated T and B lymphoma cells were found to be expressed only in CLL cells, suggesting that HCL cells represent a later stage of differentiation.
|