|
* This work was supported by a Grant-in-Aid from the Ministry of
Health and Welfare as part of a comprehensive 10-year Strategy for
Cancer Control; by a Grant for Pediatric Research 63-06 from the
Ministry of Health and Welfare; by a Grant-in-Aid for Cancer Research;
and by the Japanese Foundation for Multidiciplinary Treatment of
Cancer.
Transient myeloproliferative disorder is a hematological condition observed during the neonatal period in patients with Down syndrome [ 1] .This disorder mimics congenital leukemia and the blast cells were often reported to show characteristics compatible with those of megakaryoblasts [2] or pluripotent stem cells [3]. Although this disorder usually resolves gradually without antileukemic treatment, in some patients, leukemia develops after periods of spontaneous remission [4], raising questions about the benign origin of this disorder . It is therefore unclear if transient myeloproliferative disorder is aleukemia, preleukemia, or transient dysplasia of myelopoiesis. This important issue might be resolved by analysis of the clonality of the blast cells of this disorder. We therefore studied the clonality of blast cells in transient myeloproliferative disorder using antigen receptor genes as well as X chromosome inactivation patterns.
Patient Samples. The patients studied included three newborn infants, all of whom had Down syndrome with standard trisomy 21 (Table 1 ). They all developed transient myeloproliferative disorder during the neonatal period with more blast cells in the peripheral blood than in the bone marrow, and were followed by spontaneous resolution without antileukemic treatment. The follow-up period of these patients was from 72 days to 5 months. One out of three patients was alive at the time of this study (patient 3). The remaining two patients died while there was no evidence of leukemia. The clinical course of patient 2 was reported previously [5].
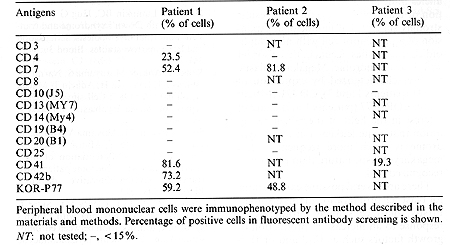
Heparinized peripheral blood was separated on a density gradient of FicollMetrizoate (Lymphoprep, Nyegaard, Oslo, Norway). The interface of mononuclear cells was subjected to immunological and DNA analysis. Reactivity of the blast cells with monoclonal antibodies against lineage-specific antibodies (Table 2) was assayed by an indirect immunofluorescence technique using a fluorescence-activated cell shorter as described before [6].
High-molecular weight DNA was extracted from the mononuclear cells according to the method described previously [6]. For the antigen receptor gene analysis, Ig1H, Cß, 1y, Cdelta, and 1delta2 probes were employed, with 6 µg of BamHI-digested DNA samples. Clonal analysis with the X-linked phosphoglycerate kinase (PGK) probe [7] was performed as described by Vogelstein et al. [8]. Table1. Clinical data of three patiens
with transient abnormal myelopoiesis 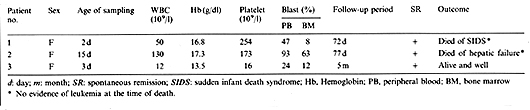
Immunological Analysis of Blast Cells. All of the samples of blast cells that we studied were positive for platelet associated antigens (CD41, CD42b, or KOR-P 77 [9]). Two out of three samples also showed positivity for CD 7 antigen. N one of three samples expressed lymphoid lineage-associated antigens such as CD 3, CD 10 or CD 19 (Table 2).
N one of the three samples of blast cells showed any rearrangements of the IgH or TCR loci using 1H, TCRß, y, and delta probes (data not shown).
94% , 99% , and 82% of the peripheral mononuclear cells were blast cells after the density gradient cell separation in patients 1, 2, and 3 respectively. After an additional digestion by restriction enzyme Hpall, DNA of the blast cells of the three patients showed complete loss of either the 1.05 kb or the 0.9 kb band, suggesting monoclonal expansion of the blast cells (Fig. 1, lanes 2, 6, and 8). In contrast, the mononuclear cells of patient 1 after spontaneous remission showed the retention of both of the alleles (Fig. 1, lane 4).
Our initial study into the lineage and clonality of blast cells using the antigen receptor genes Ig1H and TCRß, y, and delta showed no rearrangements of these loci in the blasts of any of the three patients. Table 2. Peripheral blood immunophenotyping
 As all the patients demonstrate more than 82% of blast cells in the mononuclear cell fraction, our negative findings for clonal rearrangement cannot be attributed to the low sensitivity of this assay. Furthermore, as almost all of the leukemias with lymphoid characteristics show antigen receptor gene rearrangement [10], our results suggest that the proliferating blast cells are of nonlymphoid origin. In order to study the clonality of blast cells, we employed the X chromosome linked polymorphic gene, PGK. In patient 1, mononuclear cells were compared during blastic phase and remission phase, which was achieved without therapy, for sensitivity to HpalI restriction enzyme digestion. As the results show, the absence at blastic phase, but the presence at remission phase of the allele identified at 1.05 kb indicates that mononuclear cells at blastic phase are of single clonal origin. 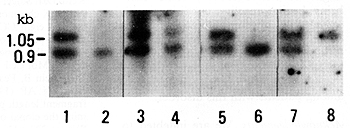
Although we could not examine the samples at remission phase in patients 2 and 3, the complete absence of one of two alleles indicates a monoclonal proliferation of blast cells. Altogether our results strongly suggest that monoclonal expansion of a progenitor cell with nonlymphoid characteristics occurs in this disorder with Down syndrome. Megakaryoblastic features characterized by expression of CD41 (patients 1 and 3), CD42b (patient 1), or KOR-P 77 (patients 1 and 2) are of interest in the light of the recent recognition that acute leukemia in Down syndrome is much more frequently of a megakaryoblastic nature than previously reco gnized [ 11] . There are several possible explanations for blast cell proliferation in this disorder . Firstly, blast cells might proliferate in response to an increase in production of growth factors such as IL-3 and/or IL-6 [12]. Secondly, hemopoietic dysregulation, including that caused by defective immunological surveillance during the neonatal period, might be responsible for this disorder. Thirdly, transient myeloproliferative disorder could be a preleukemic state. Since in the former two interpretations polyclonal proliferation of blast cells is expected, our findings strongly support the third possibility. This idea is also supported by other reports that this is due to the result of a spontaneous resolution of a malignant clone [4, 13]. Alternatively this condition might be heterogeneous, some cases being benign reactive conditions with polyclonal myeloproliferation, while others are actually preleukemic conditions. Studies on more patients with a longer survival period will clarify whether there is any heterogeneity in clonality among patients with this disorder. Acknowledgements. We are indebted to Drs. J. Singer-Sam, P. Leder, T. Rabbits, and T. W. Mak for providing the DNA probes, PGK, IgJH (lamda CH 28-6), TCR y, and TCR ß and delta respectively. We also thank Dr. S. Nakazawa for immunopheno typing data from patient 1.
|