H. Bubala, T. Jackowska, D. Michalewska, M. Ochocka, u. Radwanska,R. Rokicka Milewska, M. Rytlewska, S. Skomra, G. Sladkowska, J. Wachowiak,M. Wieczorek, E. Zelenay, D. Sonta-Jakimczuk, and A. Wozniak 1 Hämatol. Bluttransf. Vol 35 |
|
1 Departments of Hematology and Oncology, Wroclaw, Krakow, Poznan, Lublin, Warsaw, Zabrze, Poland.
Within the past 15 years, improvement in the prognosis of childhood acute lymphoblastic leukemia (ALL) has been achieved [1]. In most studies, prolonged disease-free survival has ranged from 60% to 80% [2, 3]. Nevertheless, the quality of remission is unsatisfactory in about 30% of patients, resulting in recurrence of the disease [2, 3]. The chemotherapy method elaborated by the West German Study Group (BFM) seems to offer a new way for achieving a second long-term remission in relapsed ALL [4, 5]. The aim of this study was to evaluate the results of ALL relapse therapy in children treated in seven oncology centres of the Polish Children's Leukemia/Lymphoma Study Group.
A total of 126 children (83 boys and 43 girls), aged between 6 months and 18 years (median 8.5 years), with a first relapse of ALL treated according to the BFM 1985 protocol during the years 1987-1990, were included in this study. The initial characteristics of the relapsed patients are given in Table 1. The probability of event-free survival (EFS) in the children studied was calculated according to the Kaplan-Meier method [6].
The median time from the date of obtaining the first complete remission (CR) to relapse was 24 months (3-34 months). For further analysis, the relapsed children were divided into two groups: early relapse when relapse occurred during therapy or within 6 months after completing treatment; and late relapse, when relapse occurred more that 6 months after the end of therapy. Early relapse was diagnosed in 83 patients and late relapse in 43 patients. Table 2 shows the type and time of the first ALL relapses in the children studied. A summary of the treatment response is shown in Table 3. The EFS of the children with early and late ALL relapse treated according to the BFM protocol is shown in Fig. 1. The estimated EFS in the 30th month was 52% in the late relapse group and 14% in the early relapse group (p = 0.02). The probability ofEFS for the children in the early relapse group was better with an extramedullary localization than with bone marrow involvement (45% vs. 8%, p = 0.02). The difference in the late relapse group was not statistically significant (Figs. 2, 3). Table I. Initial characteristics of the
patients 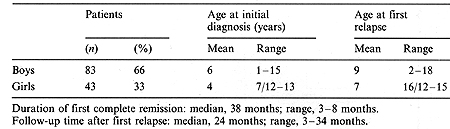
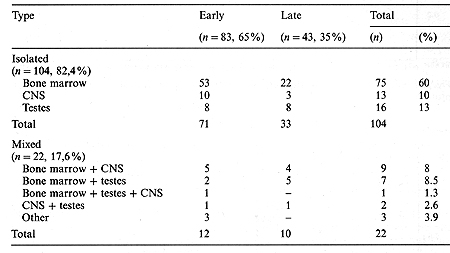 Table 3. Summary of response to treatment of the first relapse (n = 126) 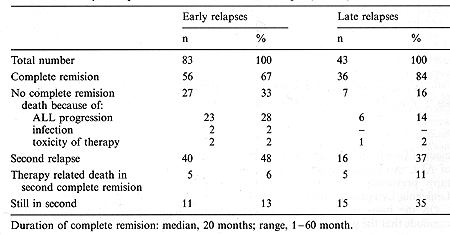
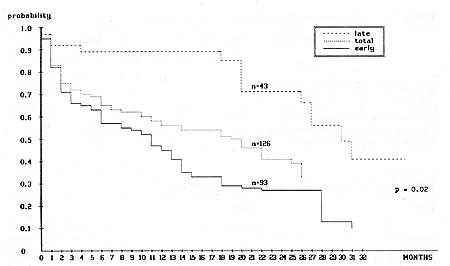 Fig. 1. Event-free survival of children with first relapse of ALL treated according to BFM protocol
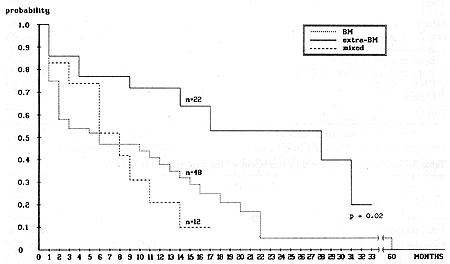 Fig.2. Event-free survival of children with first early relapse with regard to type of relapse
It was shown in our previous study that the BFM protocol produced
an improve ment of EFS in children with first relapse of ALL in
comparison with chemotherapy previously used by the Polish Leukemia/Lymphoma
Study Group [7]. On the basis of this work, we can conclude that
the probability of a second complete remission in relapsed ALL children
treated with the BFM 1985 protocol was better for the late relapse
group (p = 0.02). The results achieved in early relapse with bone
marrow involvement were not satisfactory, so some other methods
of therapy should be proposed for these patients. 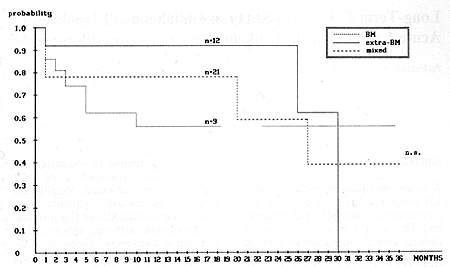 Fig.3. Event-free survival of children with first late relapse with regard to type of relapse
|