|
* Supported in part by Grant We 942/2-1 from the
Deutsche Forschungsgemeinschaft.
Severe congenital neutropenia (SCN ; Kostmann's syndrome), a disorder of myelopoiesis, is characterized by an impairment of myeloid differentiation in bone marrow with absolute neutrophil counts (ANC) below 200/µI in blood of affected patients [1-6]. This disorder was first described by Kostmann [1, 2]. Patients with SCN experience frequent episodes offever, pneumonitis, skin infections, perianal and liver abscesses, usually beginning in early infancy and often leading to fatal infections in spite of antibiotic therapy. Bone marrow morphological findings in these patients have been variable, but a maturation arrest of myelopoiesis at the promyelocyte stage is usually seen [16]. Several methods of therapy have been attempted in these patients including white cell transfusions, corticosteroids, vitamin B6, lithium, androgens, and bone marrow transplantation (BMT). To date, only BMT has resulted in partial or complete correction of the neutropenia [7, 8]. The etiology of SCN is unknown. There is no evidence for serum inhibitors of myelopoiesis or anti neutrophil antibodies in these patients. Recently, granulocyte colony-stimulating factor (G-CSF) has been purified, molecularly cloned, and expressed as recombinant protein [9,10]. It has been shown to be a potent stimulus for normal myeloid proliferation and differentiation in vitro [9,10] and in vivo [11,12]. Using bone marrow cells from SCN patients, colony-forming unit -granulocyte macrophage assays (CFU-GM) in the presence of recombinant human G-CSF (rhGCSF) demonstrated predominantly monocyte/macrophage colonies. The growth of neutrophil colonies with G-CSF as growth factor was significantly diminished ( own un published observation ) . In a previous clinical trial we investigated the effects of granulocytemacrophage-CSF (GM-CSF) in SCN patients [13] and because only one of seven patients showed any increase in circulating neutrophils we subsequently initiated a study with G-CSF. The objectives of this study were to determine the biological effectiveness of G-CSF in the treatment of SCN in order to design an optimal therapy for this fatal disease.
Patients
All patients started their treatment with 3 µg/kg per day rhG-CSF subcutaneously (s.c.). The next doselevels were 5, 10, 20, 30, 40, and 60 µg/kg per day. If no response was observed by day 14 of any dose level, patients were moved to the next dose level. Two patients who did not respond to 60 µg/kg per day s.c. were treated with 120 µg/kg per day rhG-CSF continuous intravenously ( cont. i. v. ). Patients with complete responses (ANC > 1000/µl) at any dose level were eligible for enrollment into a maintenance treatment. rhG-CSF was provided by Amgen (Thousand Oaks, CA). It was expressed in E. coli and purified to homogeneity. The rhG-CSF has a specific activity of approximately 10 high 8 U /mg protein [10]. It was endotoxin-free as judged by the rabbit pyrogen test and by the limulus amebocyte lysate assay.
The murine myeloblastic leukemia cell line, NFS-60 [14], was used to determine G-CSF levels in sera from patients with SCN. Serial dilutions of sera from SCN patients prior to rhG-CSF therapy and appropriate controls were incubated with NFS-60 cells (106/ml) for 48 h in 96-well flatbottom microtiter plates (Nunc, Roskilde, Denmark; 200 µl/well). Identical samples were also tested in the presence of the neutralizing anti-G-CSF antibody 74 A (4 µg/ml; Amgen, Thousand Oaks, USA). [³H]Thymidine (0.5 µCi/well; Amersham-Buchler, Brunswick, FRG) was added for the last 4 h of culture. Cells were then lysed and DNA harvested on glass fiber strips. Incorporated radioactivity was measured in a liquid scintillation counter. Serial dilutions of rhGCSF were used as standard, the concentrations of the samples were calculated by probit analysis from the standard curve and shown in picograms per milliliter .
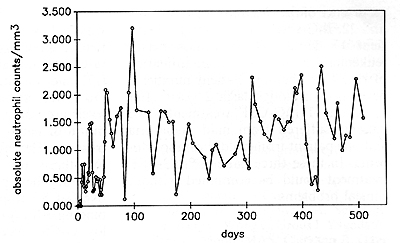
Serum Levels of G-CSF in SCN Patients Prior to rhG-CSF Therapy Sera from six patients (numbers 4, 6, 9, 12,16,28) were investigated prior to rhGCSF treatment for G-CSF activity using the NFS-60 proliferation assay. Sera from patients with SCN demonstrated significantly higher G-CSF levels than sera from controls (Table 1 ). From these proliferation data we calculated the amount of G-CSF for patients (between 150 and 670 pg/ml) and for controls (0lOO pg/ml). The addition of neutralizing antibody to these assays reduced the biologic activity of the G-CSF containing sera by between 60% and 100% (Table 1). Effects of rhG-CSF on Blood Cells The effects of rhG-CSF on blood neutrophil counts are shown in Fig. 1 and in detail for patient 8 in Fig. 2. rhG-CSF administration led, in 29 of30 patients, to an increase in ANC to levels above 1000/µl (Fig.l). The dosage needed to achieve an AN C of 1000 was between 3 µg/kg per day and 60 µg/kg per day s.c. or 120 µg/kg per day cont. i.v. Patient 9 showed only a minor response even at the Table I. G-CSF concentration of serum
samples from patient with SCN (proliferation of NFS-60 cells )  highest dose (120 µg/kg per day cont. i.v.), with an increase in ANC to only about 200/µ1. The dosage required to maintain an ANC of above 1000/µl was different in each patient: Fifteen patients required rhG-CSF dosages between 1 and 8 µg/kg per day, 9 patients between 10 and 20 µg/kg per day, and 4 patients between 40 and 60 µg/kg per day. In one patient (number 5) the rhG-CSF maintenance dosage was reduced to 0.8 µg/kg per day because of a vasculitis, most likely due to high neutrophil counts [13]. Her neutrophil counts ranged between 500 and 1000/µ1. Mean and standard deviation of all invidual measurements of neutrophil counts from all patients prior to therapy and within the time intervals 1-4,5-12, 13-52, and 53-104 weeks of rhG-CSF treatment are shown in Fig. 1. Due to the oscillation of neutrophil counts observed in all patients (see also Fig. 2) the standard deviation at a given point in time is high. However, this oscillation did not affect the beneficial clinical responses. The neutrophils did show normal functions as judged by phagocytosis, intracellular killing of staphylococci, and reactive oxygen production [15]. The absolute eosinophil counts (AEoC) did not change significantly during rhG-CSF therapy in any patient. The absolute monocyte counts (AMC) increased two- to eight-fold in the majority of patients during rhG-CSF treatment. The most dramatic increase in AMC was seen in patient 3. However, he started the rhG-CSF treatment with an 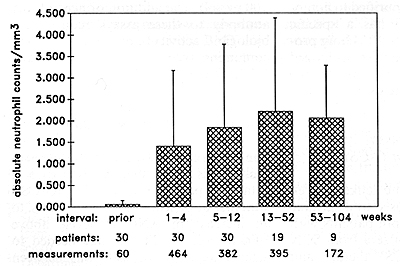
already excessively high AMC (3438/µ1), which increased further up to 24800/µ1 during the first 6 weeks of treatment. The number of CFU-GMs, myeloblasts and promyelocytes in the bone marrow during rhG-CSF treatment did not change significantly during rhG-CSF maintenance treatment.
During rhG-CSF treatment, the peptostreptococcus-caused lung infiltrates in patient 1 dramatically resolved within 6 weeks of therapy. Prior to rhGCSF treatment she received 6 weeks of i. v. antibiotics in a hospital setting. During the first 6 weeks of rhG-CSF treatment, her pulmonary situation resolved to a degree that the i. v. antibiotics could be replaced by prophylactic oral antibiotic therapy. This resolution appeared in association with the increase in neutrophils. Patient 11 suffered from a lifethreatening severe lung abscess which had destroyed most of the normal lung tissue of the left lung. She did not respond to up to 60 µg/kg per day s.c., but responded to 120 µg/kg per day cont. i. v. with an increase in ANC to above 1000/µ1. Her left lung could then be removed without complications. Interestingly enough, after the removal of the infected lung tissue, the rhG-CSF dosage could be reduced to 50 µg/kg per day s.c., maintaining an ANC above 1000/µl. In patient 12 a severe anal abscess and anal fistula which had persisted for about 1 year prior to rhG-CSF treatment in spite of surgical intervention and antibiotic treatment resolved within 3 months during rhG-CSF therapy. Patient 13 had suffered for more than 2 of the 3 years of his life from fungal liver abscesses. As soon as the neutrophils increased, the liver abscesses shrank and were not detectable anymore at a second-look laparotomy on day 90 ofrhG-CSF treatment. No new severed bacterial infections have developed in these patients.
The adverse events included necrotizing cutaneous vasculitis (patient 5), generalized vasculitis (patient 17), and mesangioproliferative glomerulonephritis (patient 22), all associated with a prompt increase in ANC and not with the dose of rhG-CSF. All three patients suffered from these side effects at the lowest dose of rhG-CSF (3 µg/kg per day). Patient 5 now receives rhG-CSF at a dose of 0.8 µg/kg per day. At this dose, she has ANC of 500-1000/µl without further recurrence of the vasculitis. In patients 17 and 22, rhG-CSF was discontinued. Patient 17 developed acute monoblastic leukemia 6 months after dicontinuation of rhG-CSF therapy. Patient number 6 developed myelodysplasia 2 years after initiation of rhG-CSF treatment. Two patients suffered from mild hematuria and one patient from mild thrombocytopenia. In these three patients, rhG-CSF treatment could be continued without clinical problems.
In this study, rhG-CSF induced an increase of blood neutrophils in 29 of 30 patients. The dose necessary to reach and maintain an ANC of above 1000/µ1 varied from patient to patient and ranged between 3 and 120 µg/kg per day. The neutrophils in the rhG-CSF-treated patients had normal functional activities as judged by in vitro functions and by clinical parameters. In four patients, there was resolution of severe bacterial infections (pneumonitis, lung abscess, liver abscess, anal abscess) resistant to i. v. antibiotic treatment prior to rhG-CSF therapy. The maintenance treatment did not lead to an exhaustion of myelopoiesis: 29 patients have now been treated for 12 months and longer. The ANC ofall patients was stable during the maintenance treatment and no increases in the dosage were necessary for maintaining the ANC during long-term treatment. The number and severity of infections decreased significantly in all patients during rhG-CSF treatment as compared to a similar time period prior to therapy. Additional SCN patients have been treated with rhG-CSF by Bonilla et al. [ 16] and showed similar increases in ANC. The hypotheses for the pathomechanism of the underlying disease include defective production of G-CSF , or defective response of neutrophil precursors to G-CSF or other hematopoietic growth factors. A defect of G-CSF production does not seem likely in light of new data which show that serum from these patients contain normal or elevated levels of G-CSF as judged by western blot analysis [17] and in vitro bioassays (Table 1). However, these endogenous G-CSF levels are not sufficient to induce maturation of neutrophil precursors in SCN patients. Therefore, the more attractive hypothesis for the genetic disposition affecting these patients involves a defective G-CSF response, either by reduced binding affinity of G-CSF to its receptor , low G-CSF receptor numbers, or defective intracellular signal transduction. Different mutations in molecules involved in the G-CSF response could explain the variations from patient to patient to achieve an ANC of 1000/µ1, and the need for pharmacological dosing (3 120 µg/kg per day) to reach this low but adequate neutrophil level supports this hypothesis. These dosages would induce an ANC of 20000-100000/µ1 in other patients [12]. There were side effects from rhG-CSF treatment in these patients. Two patients experienced a vasculitis, and one a mesangioprolifera ti ve glomerulonephri tis . Since these side effects were clearly associated with ANC of above 1000/µ1 and not with the dose of rhG-CSF, the relatively increased numbers of neutrophils have to be considered as the cause for these adverse events. The pathogenetic mechanisms of the vasculitis could be explained by infiltration of inflamed vessel walls with neutrophils and mononuclear cells and subsequent disruption of the small superficial cutaneous vessels. Deposits of immunoglobulins, complement components, or circulating unspecific immune complexes, all compensatively elevated in the blood of this patient, may have potentiated this process. The development of myelodysplasia and acute monoblastic leukemia is most likely due to the underlying disease suggesting that congenital neutropenia is a preleukemic state. This is supported by data published prior to G-CSF treatment [18]. These findings demonstrate that rhGCSF is the most promising of all available treatments for SCN. The correction of neutropenia with resultant improvement of clinical status can dramatically change the high morbidity and therefore the quality of life in these patients. The risk of death from severe bacterial infections will most likely be diminished. These results show also the feasibility of maintenance treatment with rhG-CSF for up to 2 years without exhaustion of myelopoiesis, and the benefical effects of rhG-CSF in patients with SCN .
1. Kostmann R (1956) Infantile genetic agranulocytosis. Acta Paediatr
Scand [Suppl 105] 45:1 |