St. Jude Studies XI (1984-1988) and XII (1988) * |
J. Ochs 1, W. Furman 1, V. M. Santana 1,0. H. Hustu 3,J. Sandlund 1, and W. M. Crist 1 Hämatol. Bluttransf. Vol 32 |
|
1 Department of Hematology/Oncology*,
During the past decade, evidence from clinical trials for children with acute lymphoid leukemia (ALL) indicates that improved end results can be obtained by use of intensified early therapy (1-5]. Such improvement has been especially apparent for children judged to be at higher risk of relapse based on prognostic factor analysis. Risk assessment based upon the clinical features of patients at diagnosis (e.g., age, WBC count, and race) and the biologic features of their leukemic blast cells (e.g., immunophenotype and karyotype) has made it possible to modify therapy in rational ways. Additionally, alternative methods of irradiation for the treatment of preclinical CNS leukemia have been shown to be highly effective for children with non- Bcell ALL. Newer approaches to effective CNS prophylaxis include use of socalled, triple intrathecal (IT) chemotherapy (methotrexate (MTX), hydrocortisone, cytosine arabinoside (ara-C)] given early and throughout one or more years of treatment (6]. In the St. Jude Total XI study (19841988), intensive early treatment with seven agents is being used for remission induction; this phase of therapy is followed by consolidation with high-dose MTX. During continuation treatment, we are evaluating the use of pairs of effective antileukemic drugs alternated either weekly or every 6 weeks throughout the entire duration of therapy (120 weeks). The risk of relapse is assessed by anew system based on prognostic factor analysis of data obtained in the Total X study (7]. Age, WBC count, race, DNA index, and chromosome translocation were most predictive of relapse in our Total X Study (7] and, therefore, are being used to determine risk status in the Total XI study. A summary of the efficacy and toxicity of early therapy in the Total XI trial, and plans for our next study (Total XII), form the basis of this report.
Between March 1984 and May 1988, we treated 332 consecutive children with non- B-cell ALL according to the Total XI protocol. The diagnosis of ALL is based on morphologic evaluation of Wright-stained smears of bone marrow and negative marrow myeloperoxidase preparations ( < 3% positive blasts). CNS disease is defined as greater than five mononuclear cells/mm3 on a CSF cell count and blasts seen on a cytospin preparation. Complete remission is defined as < 5% marrow blasts and signs of recovery of normal hematopoiesis. Detailed immonologic marker studies including surface immunoglobulin (Slg) evaluation are performed on all cases to rule out B-cell ALL and to define other major Table 1. Criteria for risk assignment
in Total Therapy Study XI  immunophenotypes (T, pre-B and common). All surface Ig-negative cases are included herein and detailed results of the immunologic studies will be summarized in a separate report. Flow cytometry to establish the ratio of the DNA content of leukemic versus normal cells (DNA index) and cytogenetic studies to determine the leukemic cell karyotype are successful in over 95% of cases. Results of these studies will be described separately. The clinical and biologic features that determine risk assignment are shown in Table 1, together with the estimated relative risk of treatment failure for each factor, as determined by Cox regression model analysis of data from 431 patients treated from 1979 to 1983 in our preceding Total X study. We recently published two reports summarizing the results of Total X, including details of the risk model noted above and end results at a median follow-up time of 4 years [1, 7]. The five factors noted in Table 1 were most predictive of outcome in Total X and therefore were used for risk assignment in Total XI. The presence of any two of these features resulted in a worserisk assignment; a WBC count of over lOO x 10 high 9 /liter was sufficient by itself to confer a worse risk classification. The details of therapy including drug schedule, dosage, and duration of administration are shown in Figs. 1 and 2. In brief, seven highly effective agents are used for induction treatment, given over 8 weeks; CNS prophylaxis with IT chemotherapy is started early in this phase of treatment. High-dose MTX (2 g/m² given intravenously over 2 h with 6 h of hydration and urinary alkalinization) is begun on day 43 and is repeated 1 week later. Leucovorin rescue is given at a dose of 30 mg/m² at 32,44, and 48 h, followed by 5 mg/m² at 56 and 68 h. These doses are intended to achieve total reduced folate serum concentrations above MTX concentrations at 44 hand equimolar levels until MTX concentrations are less than 0.05 µmol/liter . Three regimens were employed as continuation therapy. One-third of the better-risk patients receive conventional continuation treatment 1 (6-MP, MTX, vincristine, and prednisone) and twothirds receive treatment 2, which is experimental and consists of drug pairs rotated weekly as shown in Fig. 2. Worse-risk patients are randomized unequally between experimental treatment 2 (two-thirds) and experimental treatment 3 ( one-third). Relatively non-cross-resistant effective drug pairs with different toxicities are used and rotated either weekly (treatment 2) or every 6 weeks (treatment 3); this allows us to evaluate the effect of rapid versus slower rotation of drug pairs. Both treatments use the same drug pairs administered at identical doses. Further, this intensive continuation therapy is continued for the entire length of treatment rather than only during early therapy. Continuation therapy given is based on the patient's absolute phagocyte count (absolute number of early myeloid forms and segmented neutrophils plus monocytes). Full doses of chemotherapy are given if the absolute phagocyte count level exceeds 0.5 x 10 high 9/ liter; otherwise doses are skipped and not delayed. Central nervous system prophylaxis is continued for 1 year based on available data from studies of the Pediatric Oncology Group (ALinC 11, 12, 13), which suggest that such therapy is effective, especially for better-risk patients [6, 8]. Patients with CNS leukemia at diagnosis or those at worse risk also receive delayed cranial irradiation (2400 or 1800 cGy, respectively) after 1 year of remission. The results are sequentially analyzed and appropriate stopping rules are incorporated to avoid continuing the study unnecessarily. 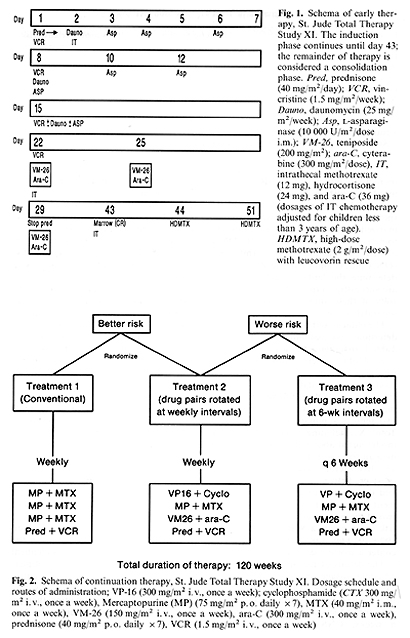
I. Patient Accrual and Risk Distribution As of 1 May 1988, 335 patients had been enrolled in the Total XI study. Only three of these children were not evaluable ( one refused therapy, two had incorrect diagnoses). Of 332 evaluable children, 107 (32%) were considered standard risk and 225 (68%) worse risk. Thirteen percent of the patients are black and 3.0% are infants ( <_ 1 year of age). Twenty-five percent are over 10 years of age, but under 18 years. Seventeen percent have a WBC count greater than lOO x 10 high 9/liter. The proportion of worse-risk patients is much higher than we had anticipated largely due to an increased number of patients with chromosomal translocations, as compared with results in the Total X study. This circumstance resulted primarily from improvements in our cytogenetic techniques during the Total X study which led to better detection of subtle translocations in Total XI. Ninety-seven percent (321/332) of our patients had successful immunologic marker studies and DNA indices performed on leukemic blast cells. Banded karyotypes were available for over 90% of the patients. The toxicity encountered during the induction phase of therapy is summarized in Table 2. It can be seen that the major problems encountered were infectious in nature and developed during periods of neutropenia. A relatively increased rate of seizures noted early in the course of Total XI was largely corrected by changing the schedule of intrathecal therapy during induction treatment. Table 2. Toxic effects during induction
therapy 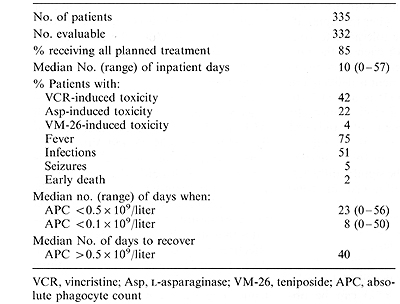
The complete remission (CR) rate for all patients is 96% (318/332). Of the 318 patients attaining CR, 106 (99%) of 107 better-risk patients and 212 (94% )/225 worse-risk patients achieved CR. Of the 14 patients who did not attain initial remission ( early failures ), 6 had primary drug resistance and 8 died of chemotherapy-related toxicity. Since we revised the induction phase of chemotherapy, the tolerance to treatment has improved and, in fact, one-third of children receive the early phase of therapy as outpatients. Ninety-seven percent of the planned doses of induction treatment have been given and 85% of patients received all planned therapy (Table 2). Ninety-six percent of children have received the two courses of high-dose MTX consolidation in the "day hospital" setting. Three hundred and eight patients have been randomized to receive continuation therapy as follows: better risk, treatment 1 (n = 37); better risk, treatment (n = 61); worse risk, treatment 2 (n = 139); worse risk, treatment 3 (n = 71). Three patients were not randomized and received other therapies after CR induction. The tolerance to continuation treatment for each of the randomized treatment regimens has been excellent and most treatment is delivered in the outpatient setting. It is too early to report the durations of remission on the three treatment regimens of Study XI (the median follow-up time is only 2 years). However, event-free survival (EFS) is estimated to be significantly better than the result obtained in Total Therapy Study X.
These results indicate that a high remission induction rate (96%)
can be ob tained without undue toxicity in children with non- BALL
using a seven-drug induction regimen that features early administration
of VM-26 and ara-C (at days 22, 25, and 26) and high-dose MTX (at
days 44 and 51). The rationale for this treatment is that very early
use of multiple effective agents may eradicate larger numbers of
leukemic cells before the emergence of drug resistance, yielding
improved end results with acceptable toxicity. Another notable feature
of the Total XI treatment is use of intensification therapy, not
only early but sustained throughout 120 weeks of continuation treatment.
It is also of interest that CNS prophylaxis for one-third of patients
who have had a lower risk of relapse does not include irradiation
and that cranial irradiation for worse-risk patients or those with
CNS disease at diagnosis is delayed for 1 year, permitting better
tolerance to systemic therapy and sparing patients who relapse early
in the bone marrow from the toxic effects of irradiation. The isolated
CNS relapse rate in Study XI is currently < 2%, and all continuation
therapy has been administered in an outpatient setting. The toxicity
has been modest in all phases of treatment. We are encouraged by
the comparatively low relapse rate in this study; at the time of
the most recent analysis, duration of event-free survival was superior
to that seen in the preceding clinical trial, although follow-up
times are too short for Study XI for statistically meaningful evaluation.
From experience in Total XI, combined with that in studies of patients
with ALL in relapse, we believe that the epidophyllotoxins VM-26
and VP-16 warrant further evaluation in clinical trials for children
with newly diagnosed ALL. Information demonstrating that highdose
MTX may be more effective if systemic exposure is optimized [7,
9] indicates that such therapy may be especially effective for patients
with better-risk ALL. Fixed dosing of each of these three agents
results in a wide variation in systemic exposure among patients
and 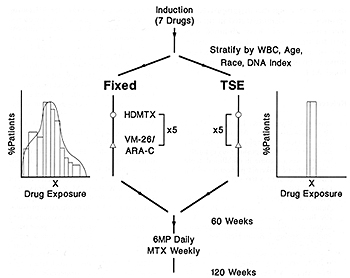
1. Dahl GV, Rivera GK, Look AT, Hustu HO, Kalwinsky DK, Abromowitch
M, Mirro J, Ochs J, Murphy SB, Dodge RK, Pui C-H (1987) Teniposide
plus cytarabine improves outcome in childhood acute lymphoblastic
leukemia presenting with a leukocyte count> 100 x 10 high 9 /L.
J Clin Onco15:1015-1021 |